In Development this Week (Vol. 144, Issue 16)
Posted by the Node, on 15 August 2017
Here are the highlights from the current issue of Development:
PLCζ ‘waves in’ mammalian oocyte activation
At fertilisation, fusion of the sperm with the oocyte activates a slew of downstream processes to kick-start embryogenesis. This ‘oocyte activation’ event induces the cortical reaction to prevent polyspermy, triggers oocyte metabolic and DNA synthesis pathways, and reactivates meiosis. In mammals, there is evidence to suggest that a phospholipase C isoform, PLCζ, initiates calcium oscillations associated with oocyte activation when delivered from sperm to egg. Hence, PLCζ is thought to be crucial for the activation process. 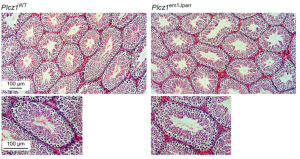 Here, on p. 2914, John Parrington and colleagues directly test this hypothesis using CRISPR/Cas9 to generate a PLCζ knockout mouse. They report that while the production and quality of sperm are unaffected in knockouts, the sperm fail to induce calcium waves at fertilisation, confirming that PLCζ is required for this event. Intriguingly, although the majority of eggs fertilised by the knockout sperm do not develop, some are able to initiate embryogenesis, albeit in a delayed manner. Remarkably, PLCζ knockout mice could father a small number of offspring that develop to term, suggesting that oocyte activation can occur via an alternative route when PLCζ-triggered calcium oscillations fail. These findings will provoke further investigation into what this alternative mechanism might be, and the PLCζ knockout will be a useful tool to study infertility in mammals.
Here, on p. 2914, John Parrington and colleagues directly test this hypothesis using CRISPR/Cas9 to generate a PLCζ knockout mouse. They report that while the production and quality of sperm are unaffected in knockouts, the sperm fail to induce calcium waves at fertilisation, confirming that PLCζ is required for this event. Intriguingly, although the majority of eggs fertilised by the knockout sperm do not develop, some are able to initiate embryogenesis, albeit in a delayed manner. Remarkably, PLCζ knockout mice could father a small number of offspring that develop to term, suggesting that oocyte activation can occur via an alternative route when PLCζ-triggered calcium oscillations fail. These findings will provoke further investigation into what this alternative mechanism might be, and the PLCζ knockout will be a useful tool to study infertility in mammals.
HIFs help make two halves
Just as in adulthood, an organism must respond to changes in its external environment during embryogenesis. Oxygen levels can fluctuate within a tissue, and animals have evolved a conserved signalling pathway to orchestrate a cell’s response to low oxygen levels (hypoxia). Critical to this pathway is the transcription factor hypoxia-inducible factor α (HIFα), which is degraded in conditions of normoxia. However, in low oxygen, it binds with its partner HIFβ to hypoxia-response elements and activates downstream genes that are important for a cell to cope with oxygen depletion. 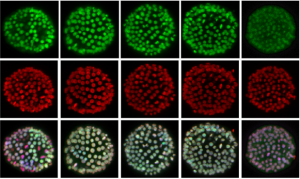 On p. 2940, Yi-Hsien Su and colleagues demonstrate that the hypoxia signalling pathway is active in the early sea urchin embryo, in a graded manner that mirrors the emerging dorsoventral axis. They report that while hifα mRNA is distributed uniformly throughout the embryo, the protein is stabilised at the dorsal side, and degraded more ventrally. They found that HIFα protein restricts nodaltranscripts to the ventral ectoderm only, and that the dorsoventral axis is affected by artificial perturbation of HIFα levels. Interestingly, they also found evidence for an intrinsic hypoxia gradient in embryos, which may be a forerunner to dorsoventral patterning. Together, these results provide a fascinating insight into the question of how environmental signals can impact early development.
On p. 2940, Yi-Hsien Su and colleagues demonstrate that the hypoxia signalling pathway is active in the early sea urchin embryo, in a graded manner that mirrors the emerging dorsoventral axis. They report that while hifα mRNA is distributed uniformly throughout the embryo, the protein is stabilised at the dorsal side, and degraded more ventrally. They found that HIFα protein restricts nodaltranscripts to the ventral ectoderm only, and that the dorsoventral axis is affected by artificial perturbation of HIFα levels. Interestingly, they also found evidence for an intrinsic hypoxia gradient in embryos, which may be a forerunner to dorsoventral patterning. Together, these results provide a fascinating insight into the question of how environmental signals can impact early development.
A fishy response of transposons to demethylation
DNA methylation is an epigenetic mechanism that promotes heterochromatin formation, silences imprinted loci, the X chromosome, repeats and transposable elements. The idea that DNA methylation also represses differentially expressed genes has been challenged by experiments showing that loss of genomic methylation, either during early embryonic development or in mutants, does not result in a burst of gene activation. 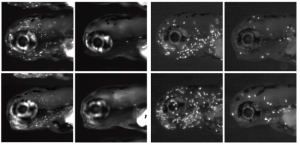 On p. 2925, work by Kirsten Sadler and co-workers confirms and extends the model that DNA methylation functions primarily as a gatekeeper for transposons. They report that mutants with a hypomethylated genome upregulate interferons, leading to the recruitment and expansion of immune cells in the developing larva. Rather than being directly due to derepression of interferon genes in the demethylated state, interferon production is stimulated by the detection of nucleic acids in the cytosol of a cell. This normally indicates the presence of a virus, but the mutants used in the study were not infected. So, what elicits this response? The authors reveal that the aberrant transcription of transposable elements caused by demethylation results in the production of cytosolic DNA that triggers the antiviral response. This mechanism could act as an early-warning system, allowing cells in the developing embryo with widespread epigenetic abnormalities to be put under immune surveillance so they can be rapidly eliminated if necessary.
On p. 2925, work by Kirsten Sadler and co-workers confirms and extends the model that DNA methylation functions primarily as a gatekeeper for transposons. They report that mutants with a hypomethylated genome upregulate interferons, leading to the recruitment and expansion of immune cells in the developing larva. Rather than being directly due to derepression of interferon genes in the demethylated state, interferon production is stimulated by the detection of nucleic acids in the cytosol of a cell. This normally indicates the presence of a virus, but the mutants used in the study were not infected. So, what elicits this response? The authors reveal that the aberrant transcription of transposable elements caused by demethylation results in the production of cytosolic DNA that triggers the antiviral response. This mechanism could act as an early-warning system, allowing cells in the developing embryo with widespread epigenetic abnormalities to be put under immune surveillance so they can be rapidly eliminated if necessary.
PLUS…

An interview with Jenny Nichols
The 2017 BSDB Cheryll Tickle Award winner talks to us about her career in science, the importance of collaboration, and the similarities between playing musical instruments and manipulating embryos.
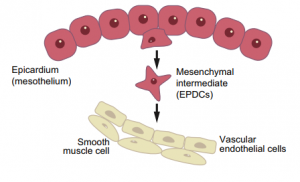
Wilms’ tumour 1 (WT1) in development, homeostasis and disease
This Primer summarises our current understanding of the diversity of WT1 functions in mammalian tissues and organs and how WT1 mutations can lead to disease.
Cellular and molecular mechanisms coordinating pancreas development
This Review describes the gene regulatory networks, signaling pathways, morphogenetic movements and cell dynamics underlying organogenesis of the mammalian pancreas.


 (No Ratings Yet)
(No Ratings Yet)