Manchester PhD position on neuronal development, ageing & degeneration
Posted by Andreas Prokop, on 17 October 2017
Closing Date: 15 March 2021
The University of Manchester, 2018/19 BBSRC DTP PhD Project
Understanding tubulin regulation during neuronal development, ageing and degeneration
Axons are slender, up-to-a-meter long, cable-like extensions of neurons which form the nerves and nerve tracts that wire our bodies and brain. These delicate cellular structures have to be maintained for an organism’s life time and are often the first to be affected in ageing, injury and neurodegeneration. To understand such conditions and identify ways to improve axon maintenance and regeneration, we study the regulation of microtubules (MTs) which form parallel bundles running all along axons to form their structural backbones and life-sustaining transport highways.
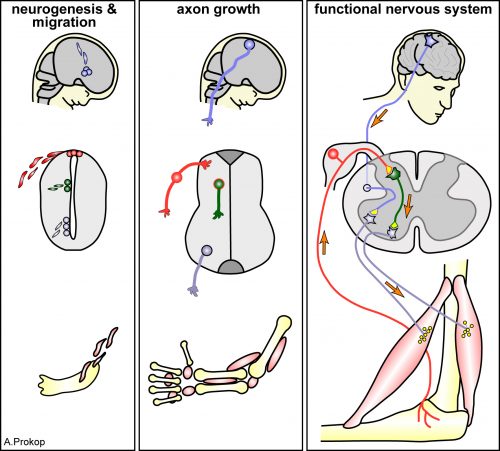
On this project, you will study how the polymerisation of MTs within these bundles is regulated to drive axon growth and prevent senescence during ageing. The key question is how tubulins (i.e. the building blocks of MTs) are made available and continuously supplied in the narrow axons, up to a meter away from the cell body. This fascinating topic is most relevant to axon biology and pathology but surprisingly little understood. Your pioneering work will be based on our recently published mechanistic model of axonal tubulin supply and MT polymerisation, deduced from our own data and general knowledge in the field [Ref.1]. You will use cutting edge methodologies to study (1) contributions made by axonal transport and local tubulin translation, (2) roles of the chaperone machinery of tubulin assembly, and (3) mechanisms of tubulin storage and gene expression regulation.
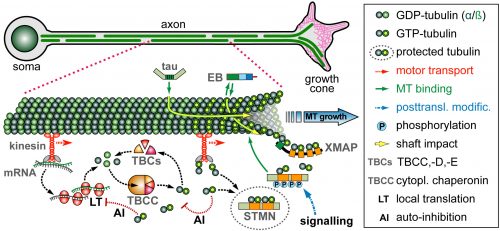
For your studies, you will use neurons of the fruit fly Drosophila, which provide uniquely powerful genetic and cell biological means in order to efficiently generate new understanding that can then be applied to higher animals [Ref.2; LINK]. You will be able to capitalise on expertises of the supervisory team: the host group (Andreas Prokop) has long-standing experience with MT regulation and the Drosophila neuron model, the first co-supervisor’s group (Thomas Waigh) with high resolution imaging and quantitative approaches [Ref.3; Ref.4], and the second co-supervisor’s group (Mark Ashe) with RNA visualisation and processing [Ref.5].
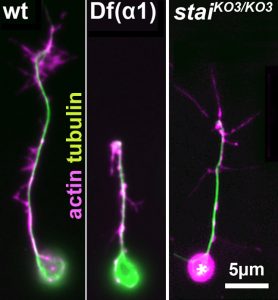
Your transferable and experimental skill training will include genetics, cell biology, molecular biology, imaging techniques (live, high resolution, axonal transport, spatial detection of RNA and translational activity), quantitative analyses and modelling, as well as expertise in the important research areas of cytoskeleton and neurobiology. Finally, Andreas Prokop is an expert in science communication [Ref.6] providing further training opportunities important for your future career.
Information and applications:
- For more information, contact Prokop@manchester.ac.uk
- To find the project, go to this LINK (search for “Prof. Prokop” in the supervisor field)
- To apply, go to this LINK
- Deadline for applications is Fri 2nd of Nov. 2017, 5pm
- Interviews are held on Tue/Wed, 9th/10th Jan. 2018
- Offers will be confirmed in mid-Feb. 2018
References
[1] Voelzmann, A., Hahn, I., Pearce, S., Sánchez-Soriano, N. P., Prokop, A. (2016). A conceptual view at microtubule plus end dynamics in neuronal axons. Brain Res Bulletin 126, 226-37
[2] Prokop, A., Beaven, R., Qu, Y., Sánchez-Soriano, N. (2013). Using fly genetics to dissect the cytoskeletal machinery of neurons during axonal growth and maintenance. J. Cell Sci. 126, 2331-41
[3] Kenwright, D.A., Harrison, A.W., Waigh, T.A., Woodman, P.G., Allan, V.J. (2012) First-passage-probability of active transport in live cells, Physical Review E, 86, 031910
[4] Georgiades, P., Allan, V.J., Dickinson, M., Waigh, T.A. (2016) Reduction of coherent artefacts in super-resolution fluorescence localisation microscopy, Journal of Microscopy, 264, 3, 375-383
[5] Sfakianos, A. P., Whitmarsh, A. J., Ashe, M. P. (2016). Ribonucleoprotein bodies are phased in. Biochemical Society Transactions 44, 1411-1416
[6] Illingworth, S., Prokop, A. (2017). Science communication in the field of fundamental biomedical research


 (1 votes)
(1 votes)