Nerves read the electrical topography of their microenvironment in making growth decisions
Posted by Mike Levin, on 29 June 2015
A really interesting recent paper on bioartificial limbs underscored the prospect of transplantation for problems in regenerative medicine. One key issue facing transplant technology is establishing appropriate innervation to the host. What factors control the amount of nerve emanating from an organ graft and the paths that this innervation takes? Alongside the familiar diffusible signaling factors and extracellular matrix components functions another important signaling modality: bioelectricity.
The ability of nerves to respond to physiological-strength extracellular electric fields has been long known. My lab studies a different aspect of bioelectricity: signaling via endogenous resting potentials across the plasma membrane of all cells (not just neurons). We have found that the spatial distribution of these voltage states (anatomical Vmem gradients) are instructive influences for stem cell function, regenerative response, organ-level reprogramming, and metastasis. Recently however, we used the Xenopus laevis embryo model to show another role for bioelectric gradients – the control of ectopic innervation.
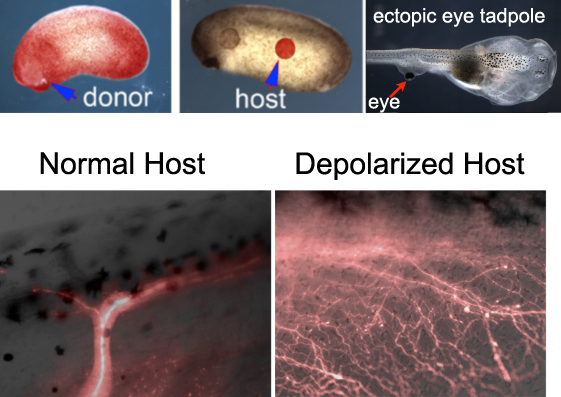
Douglas Blackiston, a post-doc in our group, was interested in the plasticity of the brain. He showed that ectopic eyes transplanted to the sides of tadpoles enabled the resulting animals to learn in behavioral assays even when the primary eyes were absent. Apparently the brain had little difficulty recognizing an ectopic organ on the animal’s back as providing useful visual data; the tadpole brain was able to adjust its behavioral programs as needed, despite the fact that this was a novel anatomical configuration (not experienced during evolution) and that the eye became connected to the spinal cord, not the brain.
Transplanting eyes from RFP-labeled hosts allowed us to see the optic nerves emanating from the transplanted eye; normally there was just one or two main fibers.
The big surprise came when he used a technique we had previously developed for the study of bioelectrically-induced melanoma to depolarize a fraction of cells in the host animal. This strategy relies on the use of a drug that opens chloride channels and allows negative ions to leave cells expressing that channel, thus depolarizing them. When eyes were transplanted into hosts depolarized by this method, instead of one major nerve bundle we observed a massive innervation. A set of molecular-genetic experiments using Doug’s meticulous transplantation assay further showed that:
- 1) the host’s endogenous innervation did not change after depolarization: depolarization appears to be a signal that is particularly attended to by neurons that somehow know they are not in their normal location; but,
- 2) eyes grafted to their normal location did not hyperinnervate, suggesting that merely being surgically perturbed is not sufficient to fool these cells.
- 3) whether the eye came from the same animal or a different donor made no difference: this was not an effect based on self/not-self detection.
- 4) a range of methods (using chloride, potassium, or sodium) could be used to induce or rescue hyperinnervation, demonstrating that the phenotype is not an off-target effect of the drug, nor dependent on that one particular channel or even the chloride ion: the cells are sensitive to Vmem itself, regardless of which ion is used to achieve it.
- 5) depolarizing the donor eye did not induce hyperinnervation, suggesting that this is a non-cell-autonomous effect: the cells are reading the surrounding environment’s Vmem levels, not their own, in making growth decisions.
- 6) The ability of neurons to respond to depolarization of their neighbors required 2 things: serotonergic signaling through 5HT-R1,2, and gap-junctional communication.
These data suggested a model of the movement of serotonin among cells via gap junctions, under control of bioelectric gradients, as a signal that regulates neural extension and pathfinding. In this aspect, these data are similar to previous findings that bioelectric control of serotonin and gap junctional connectivity regulates left-right patterning, metastasis, and tumorigenesis. However, they raise numerous new questions. First, precisely how do neurons know when they are in the wrong location – what mechanism allows just the ectopic nerves to overgrow, leaving endogenous innervation completely normal? Second, what is the encoding used by the transmitted serotonin – are absolute levels important, or is the movement pulsatile (rate encoding), or spatially-patterned? The latter issue is especially difficult to address at this time because serotonin is too small to be fluorescently labeled without drastically affecting its movement through transporters and gap junctions. Finally, could strategic misexpression of ion channels, serotonergic transporters, and gap junction proteins be used to allow fine control of neural connectivity in transplantation contexts? New advances in developmental optogenetics, together with existing tools developed by labs working on neurotransmitter signaling, could allow sculpting of nerve growth from transplanted organs, whether bioengineered or harvested from living hosts.
Since Vmem regulation and serotonergic signaling appear to be involved in guidance of mammalian neurons as well, these data likely have implications for biomedicine. Indeed, a plethora of ion channel drugs exist, many of which are already approved for use in human patients for other indications. These form an “electroceutical” toolkit which could be richly exploited for the control of neural and other cell behavior in regenerative medicine and bioengineering. Our lab is currently working on this goal in several models, as well as investigating the cognitive (behavioral) implications of massively increasing neural connections from transplanted sensory organs in Xenopus. Stay tuned!


 (6 votes)
(6 votes)