Stem cell makes its own niche: the story behind the paper
Posted by MBBaghdadi, on 7 July 2018
In our recent paper published in Nature, we unravel a new mechanism of an extracellular matrix protein secreted by muscle satellite (stem) cells, thereby playing the unusual role of acting as a signaling molecule to maintain the stem cell population. Here, I share the story behind this discovery and discuss the questions related to niche regulation.
In a ChIP-sequencing screen in myogenic cells performed by the Tajbakhsh and Stunnenberg labs (Castel et al., 2013) to identify direct targets of nuclear NOTCH (NICD) and its downstream effector RBPJ, we found an enrichment of extracellular matrix proteins, among which were specific collagens genes including Collagen 5 (Col5a1 and Col5a3) and Collagen 6 (Col6a1 and Col6a2). I was in the first year of my PhD when Dr. Philippos Mourikis, an ex post-doc in the Tajbakhsh lab, asked me to help with some collagen immunostainings on embryonic muscles over-expressing NICD (E17.5 Myf5Cre; R26NICD) (Mourikis et al., 2012). We nicely showed that both COLV and COLVI secretion were upregulated upon Notch induction.
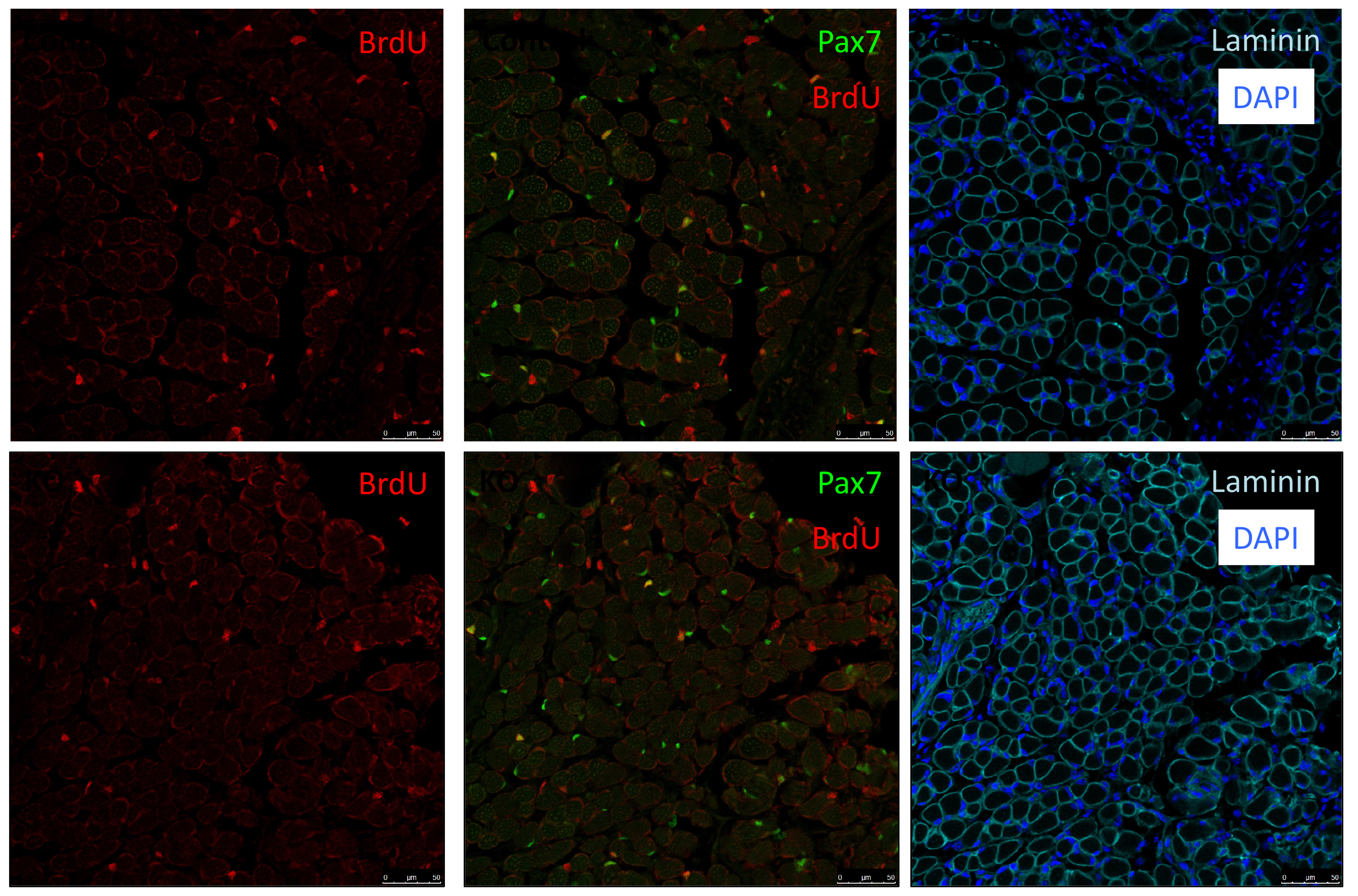
As Col5a3 gene was the most responsive gene to Notch activity, both in vitro and in vivo, we thought that Col5a3 KO mice might present a muscle phenotype (Huang et al., 2011). In collaboration with Dr Guorui Huang from the Greenspan lab, we analyzed the number of satellite cells (PAX7+) in adult and postnatal P8 pups muscle sections. Unfortunately, we could not observe any alterations in stem cell number nor proliferation capacity (BrdU pulse).
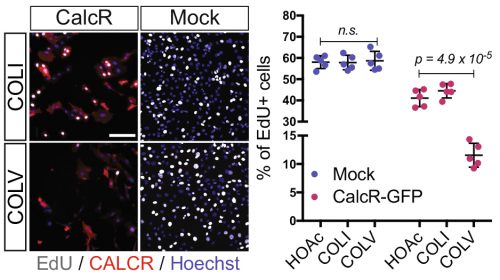
As my initial PhD project was on hold due to mice breeding issues, we decided that I could perform a simple culture experiment to close up the collagen story. We performed two sets of experiments: in the first, we added different collagens in soluble form on cells plated on gelatin-coated dishes; in the second, cells were cultured directly on collagen-coated dishes. Surprisingly, only COLV in its soluble form retained cells in a more stem-like state delaying their proliferation and differentiation. With these unexpected results, we realized that COLV might be acting as a signaling molecule rather than a biomechanical cue. As collagen molecules are known to bind to specific receptors such as Integrins or Discoidin Receptors (Leitinger, 2011), we tested whether their inhibition could abrogate COLV anti-myogenic effect. To our disappointment, COLV was still able to delay myoblast differentiation even when these receptors were inhibited.
After looking at the results from several angles, we came to the conclusion that we lacked a mechanism and we should probably wrap up the story with what we had, so that I could go back to my initial PhD project. With a sense of unfinished business, we started gathering the data into figure formats. We were then struck by an old RT-qPCR assay on satellite cells that were treated with the different collagens, where Calcitonin receptor (CalcR), a known satellite cell quiescence marker, was upregulated more than 20-fold. We remembered that CALCR is a G-protein couple receptor (GPCR) and found in vitro evidence that some collagens could indeed bind to GPCRs (Luo et al., 2011; Paavola et al., 2014). This was a major turning point in the project, and the path was wide open for detailed mechanistic studies.
In parallel on the other side of the world, our collaborator Dr. So-Ichiro Fukada in Osaka had just a few weeks earlier published a very nice study showing that specific ablation of Calcr in muscle stem cells led to their spontaneous exit from the quiescent niche (Yamaguchi et al., 2015). Using their retroviral tools to overexpress CALCR and Calcr null muscle stem cells, we were able to demonstrate that CALCR was necessary to respond to COLV. This was too exciting, my other project had to wait!
On the other hand, these results were puzzling…CalcR already has its ligand: Calcitonin hormone produced by the parathyroid gland, suggesting that satellite cell quiescence is under systemic control. Why would the regulation of muscle stem cells that are dispersed throughout muscle masses in the body – and that would be solicited with different kinetics – be under systemic control? Can COLV act as the local ligand of CalcR for muscle stem cells?
Meanwhile, our first priority was to assess whether the loss of function of COLV specifically in satellite cells using Tamoxifen-inducible Tg:Pax7-CreERT2; Col5a1flox mice could affect their behavior. Another unexpected surprise was the observation that only 18 days following recombination, satellite cells escaped the quiescent niche, differentiated and fused to the pre-existing fibres.
Another consideration is the source of collagen in skeletal muscle – being predominantly from interstitial myofibroblasts, which deposit collagen between myofibres (Zou et al., 2008). We also found expression of Col5a-1, -2, and -3 in other cell types present in the muscle; namely Fibro-Adipogenic Progenitor (FAPs) and resident macrophages.
So why does this source of COLV not compensate for the loss of satellite cells-produced COLV?
Possible explanations include the basal membrane (BM) forming a physical diffusion barrier between the satellite cells and the interstitial space – rendering them blind this source of COLV. Although the myofibre that is in intimate contact with satellite cells under the BM could be a provider, we showed that myofibres do not express detectable COLV to sustain satellite cell quiescence.
Another possibility is that COLV produced by satellite cells has a specific configuration. In our siRNAs experiments on isolated myofibers, we observed that targeting of the Col5a3 isoform phenocopied siRNA against Col5a1, strongly suggesting that the active triple helix is the a3-COLV isoform composed of both a1 and a3 chains as an [α1(V)α2(V)α3(V)] heterotrimer. Although the presence of soluble COLV is difficult to demonstrate in vivo, we favoured the possibility that COLV produced by satellite cells rapidly binds to CALCR upon secretion, rather than being integrated in the collagen network. This would then explain why loss of COLV function exhibited a phenotypic loss of satellite cells within such a short period.
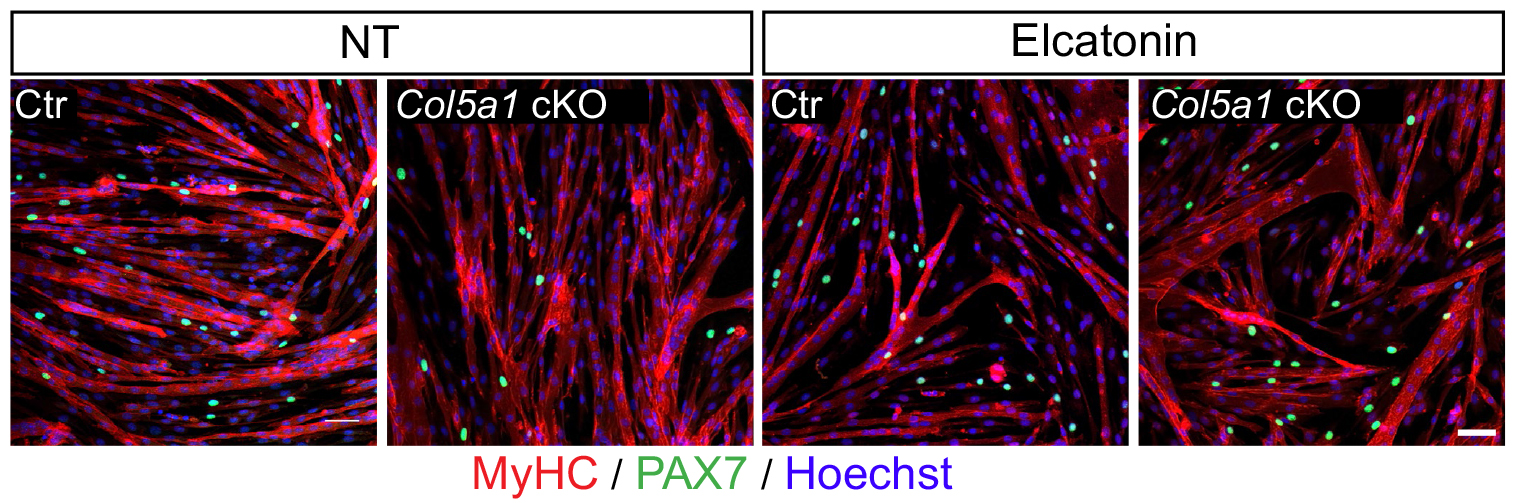
We then needed to demonstrate that the newly discovered Notch/COLV/CALCR axis is functional for the maintenance of quiescent cells in vivo. To do so, we injected Col5a1 cKO (Tg:Pax7-CT2; Col5a1flox) mice subcutaneously with a derivative of the known CALCR ligand calcitonin (Elcatonin). Interestingly, exogenous Elcatonin administration compensated for the lack of COLV and prevented their exit from quiescence, thereby validating our results linking COLV and CALCR. It also told us that endogenous calcitonin was not bioavailable in the muscle to rescue the loss of COLV. Interestingly, thyroidectomy in adult individuals does not show any striking overall pathological consequences (Russell et al., 2014); it would be interesting to study satellite cell dynamics in mice suffering from hyper/hypothyroidism.
An interesting outcome of the rescue experiments was that Elcatonin administration in control mice also showed an improvement in self-renewal capacity, suggesting that this could be a good therapeutical candidate for myopathies.
In addition, it has been shown that the satellite cell population is heterogenous according to the muscle anatomical location and even within a single muscle (Rocheteau et al., 2012; Sambasivan and Tajbakhsh, 2015). Therefore, we cannot exclude the possibility that some satellites cells do indeed respond to calcitonin stimulation. Our results showing that Elcatonin administration induces a deeper quiescent state could support this notion. In addition, CALCR is expressed in about 80% of satellite cells, suggesting that 20% of them are most likely not under direct Notch/COLV/CALCR control.
While exploring the quiescence in muscle stem cells, we learned that when studying the role of collagens one should consider not only the traditional mechanotransduction role of these molecules, but also their potential role as signaling agents. Given that neural and intestinal, and perhaps other stem cells, are maintained by Notch signaling, it is possible that this model operates in stem cells located in other tissues and organs.
Meryem B. Baghdadi
REFERENCES
Castel, D., Mourikis, P., Bartels, S.J., Brinkman, A.B., Tajbakhsh, S., and Stunnenberg, H.G. (2013). Dynamic binding of RBPJ is determined by Notch signaling status. Genes Dev27, 1059-1071.
Huang, G., Ge, G., Wang, D., Gopalakrishnan, B., Butz, D.H., Colman, R.J., Nagy, A., and Greenspan, D.S. (2011). alpha3(V) collagen is critical for glucose homeostasis in mice due to effects in pancreatic islets and peripheral tissues. The Journal of clinical investigation121, 769-783.
Leitinger, B. (2011). Transmembrane collagen receptors. Annual review of cell and developmental biology27, 265-290.
Luo, R., Jeong, S.J., Jin, Z., Strokes, N., Li, S., and Piao, X. (2011). G protein-coupled receptor 56 and collagen III, a receptor-ligand pair, regulates cortical development and lamination. Proc Natl Acad Sci U S A108, 12925-12930.
Mourikis, P., Gopalakrishnan, S., Sambasivan, R., and Tajbakhsh, S. (2012). Cell-autonomous Notch activity maintains the temporal specification potential of skeletal muscle stem cells. Development139, 4536-4548.
Paavola, K.J., Sidik, H., Zuchero, J.B., Eckart, M., and Talbot, W.S. (2014). Type IV collagen is an activating ligand for the adhesion G protein-coupled receptor GPR126. Sci Signal7, ra76.
Rocheteau, P., Gayraud-Morel, B., Siegl-Cachedenier, I., Blasco, M.A., and Tajbakhsh, S. (2012). A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell148, 112-125.
Russell, F.A., King, R., Smillie, S.J., Kodji, X., and Brain, S.D. (2014). Calcitonin gene-related peptide: physiology and pathophysiology. Physiological reviews94, 1099-1142.
Sambasivan, R., and Tajbakhsh, S. (2015). Adult skeletal muscle stem cells. Results and problems in cell differentiation56, 191-213.
Yamaguchi, M., Watanabe, Y., Ohtani, T., Uezumi, A., Mikami, N., Nakamura, M., Sato, T., Ikawa, M., Hoshino, M., Tsuchida, K., et al.(2015). Calcitonin Receptor Signaling Inhibits Muscle Stem Cells from Escaping the Quiescent State and the Niche. Cell reports13, 302-314.
Zou, Y., Zhang, R.Z., Sabatelli, P., Chu, M.L., and Bonnemann, C.G. (2008). Muscle interstitial fibroblasts are the main source of collagen VI synthesis in skeletal muscle: implications for congenital muscular dystrophy types Ullrich and Bethlem. Journal of neuropathology and experimental neurology67, 144-154.


 (4 votes)
(4 votes)