In Development this week (Volume 137, Issue 18)
Posted by Seema Grewal, on 24 August 2010
Non-muscle myosin II translates cilia polarity
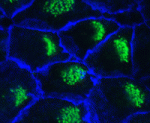
In the brain, cilia on the multiciliated ependymal cells that line the brain ventricles circulate cerebrospinal fluid over the brain surface. To generate this directional fluid flow, the ependymal cell cilia and their basal bodies must be orientated in one direction. This ‘rotational’ polarity is regulated by the planar cell polarity (PCP) pathway. Recent reports have revealed that the basal bodies are also localised at the anterior of the ependymal cells but how is this ‘translational’ polarity established? Using a new method for time-lapse imaging of ventricular walls, Kazunobu Sawamoto and co-workers now show that, in mice, the anterior migration of basal bodies in the apical cell membrane during ependymal cell differentiation establishes translational polarity (see p. 3037). Inhibition of the PCP protein dishevelled 2, which disrupts rotational polarity, does not affect translational polarity, the researchers report. Instead, their pharmacological and genetic studies identify non-muscle myosin II as a key regulator of translational polarity. Thus, different mechanisms regulate the orientation and distribution of basal bodies in ependymal cells.
SNP links Dlx gene regulation to autism
Several neurodevelopmental disorders, including autism, have been linked to the aberrant development of γ-aminobutyric acid (GABA)-expressing interneurons in the mammalian forebrain. Dlx homeobox genes control the development of these interneurons and now, on p. 3089, Marc Ekker and colleagues report that a rare, autism-associated single-nucleotide polymorphism (SNP) in an ultraconserved regulatory element (I56i) in the DLX5/DLX6 bigene cluster affects Dlx5/Dlx6 regulation in the mouse forebrain. The researchers show that the SNP, which lies in a functional protein binding site, reduces I56i enhancer activity in the developing mouse forebrain and in adult GABAergic interneurons. Notably, Dlx proteins have a reduced affinity for the variant I56i protein binding site in vitro, they report, which reduces the transcriptional activation of the enhancer by Dlx. The researchers propose, therefore, that impaired I56i enhancer activity by the SNP could affect the auto- or cross-regulation of the DLX5/DLX6 bigene cluster, thereby disrupting cortical interneuron development and contributing to the developmental abnormalities that underlie autism.
Symmetric neural progenitor divisions Notch up
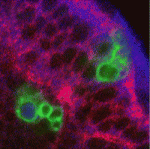
During development, the balance between neural stem cell self-renewal and differentiation is carefully controlled to ensure that the correct number of neurons is produced to build functional neural networks. In the Drosophila optic lobe, as in the mammalian cerebral cortex, neuroepithelial (NE) cells initially divide symmetrically to expand the stem cell pool, before switching to asymmetric division to generate neurons. Andrea Brand and colleagues now report that Notch regulates this important cell fate transition (see p. 2981). By comparing the transcriptomes of microdissected NE cells and neuroblasts, the researchers show that Notch signalling pathway members are preferentially expressed in NE cells. Notch mutant cells are extruded from the neuroepithelium and undergo premature neurogenesis, they report. Furthermore, a wave of proneural gene expression transiently represses Notch activity in NE cells to enable the transition from symmetrically dividing NE cell to asymmetrically dividing neuroblast. This progression resembles that seen in the vertebrate cerebral cortex, leading the researchers to propose that neurogenesis regulation could be conserved between these two systems.
Changing identities: neuronal transdifferentiation
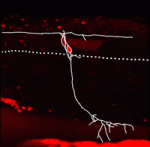
Traditionally, cellular differentiation is thought to be an irreversible commitment to a given cell identity. So, for example, differentiated neurons cannot generate new cells or adopt new identities. Now, however, Melissa Wright and colleagues provide evidence for the transdifferentiation of dorsal root ganglia (DRG) sensory neurons in zebrafish larvae (see p. 3047). Using time-lapse microscopy, the researchers track DRG neurons in wild-type zebrafish and in zebrafish mutant for the nav1.6 voltage-gated sodium channel. Some DRG neurons migrate ventrally from their normal position and then adopt a phenotype characteristic of sympathetic neurons in both types of larvae, they report, but more DRG neurons transdifferentiate in the mutant larvae. Furthermore, although the loss of sodium channel expression promotes the migration of DRG neurons, once in a new environment, these neurons transdifferentiate regardless of sodium channel expression. Thus, the researchers conclude, differentiated sensory neurons retain the plasticity needed to transdifferentiate when challenged by a new environment, a finding that suggests new strategies for the treatment of nervous system diseases.
Heartfelt responses to opposing FGF/BMP signals
Congenital heart disease – the commonest type of human birth defect – is the result of abnormal early heart development. In this issue, two papers investigate how opposing fibroblast growth factor (FGF) and bone morphogenetic protein (BMP) signals control the differentiation of the secondary heart field (SHF) and anterior heart field (AHF) cardiac progenitors during early vertebrate heart development.
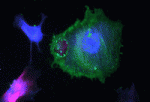 On p. 3001, by isolating and culturing chick SHF mesoderm, which forms the myocardium and smooth muscle of the heart’s arterial pole (the outflow region of the heart), Mary Hutson and colleagues show that this tissue contains stem cells that can differentiate into myocardium, smooth muscle and endothelial cells. By treating SHF (arterial pole) progenitor cultures with combinations of growth factors and inhibitors, the researchers show that BMP promotes myocardial differentiation but not proliferation of the arterial pole progenitors, whereas FGF promotes their proliferation and smooth muscle cell differentiation but inhibits myocardial differentiation. These and other results indicate that myocardial differentiation of the SHF progenitors requires BMP signalling and downregulation of the FGF/ERK pathway and suggest that the FGF pathway maintains the SHF stem cell pool early but promotes smooth muscle cell differentiation later.
On p. 3001, by isolating and culturing chick SHF mesoderm, which forms the myocardium and smooth muscle of the heart’s arterial pole (the outflow region of the heart), Mary Hutson and colleagues show that this tissue contains stem cells that can differentiate into myocardium, smooth muscle and endothelial cells. By treating SHF (arterial pole) progenitor cultures with combinations of growth factors and inhibitors, the researchers show that BMP promotes myocardial differentiation but not proliferation of the arterial pole progenitors, whereas FGF promotes their proliferation and smooth muscle cell differentiation but inhibits myocardial differentiation. These and other results indicate that myocardial differentiation of the SHF progenitors requires BMP signalling and downregulation of the FGF/ERK pathway and suggest that the FGF pathway maintains the SHF stem cell pool early but promotes smooth muscle cell differentiation later.
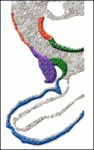
On p. 2989,, Eldad Tzahor and colleagues provide further insights into how opposing BMP and FGF signals regulate cardiogenesis by studying the differentiation of chick AHF progenitors, which contribute to the right ventricle and to the arterial pole. By perturbing signalling pathways in vitro and in vivo, the researchers show that, as in SHF progenitors, BMP promotes myocardial differentiation of AHF progenitors by blocking FGF/ERK signalling and that FGF signalling prevents their premature myocardial differentiation. They also show that BMP4 induces the expression of several neural crest-related genes and that cranial neural crest cells are required for BMP-dependent myocardial differentiation of the AHF progenitors. Thus, Tzahor and colleagues suggest, BMP and FGF signalling pathways coordinate the balance between the proliferation and differentiation of cardiac progenitors in the AHF through regulatory loops that act in multiple tissues.


 (1 votes)
(1 votes)