New strings for the puppeteer of evolution
Posted by Mark Rebeiz, on 30 April 2021
Sarah Jacquelyn Smith, Lance Davidson and Mark Rebeiz
One of the biggest mysteries in the developmental evolution field is the puzzle of how new morphological structures come about. If you think about it, every anatomical structure in the multicellular world was new at some point in time. And yet, we currently only have a rough picture of how insect wings, beetle horns, or turtle shells initially evolved. There are multiple ways that this question can be answered, and it is an exciting time to be studying how genetic programs of gene expression translate into the physical manifestations of development that form new tissue configurations. In studying one such novelty in Drosophila, we recently learned that there is more than meets the eye to generating extreme deformations in cellular shape1. We also learned to listen more carefully to our collaborators…
Novelty in Drosophila genital traits
Reproductive structures are notorious for their rapid evolution among internally fertilizing species2. The chitinous cuticle shapes in the genitalia of Drosophila melanogaster are indeed quite different from its nearest relatives. In fact, these differences in genital morphology are used to discriminate this model organism from its closest relatives3. Namely, the posterior lobe is a recently evolved hook-shaped structure which is attached to a cuticular plate known as the lateral plate (Fig. 1). The posterior lobe is highly divergent in size and shape in species that possess this structure and is absent in more distantly related Drosophila species (Fig. 1), which offer a useful reference for understanding how the genitalia likely developed before the evolution of this structure. Because the posterior lobe is present in the highly tractable Drosophila melanogaster, we can exploit the bountiful genetic tools of this model organism to examine and dissect its development. Our previous work had suggested that many genes expressed in the posterior lobe were re-deployed, or ‘co-opted’ from another organ system, the posterior spiracle4, which is an extension of the larval tracheal system. Identifying genes expressed in a structure however doesn’t tell you much about how it is built, and thus studies of genetic control mechanisms must be complemented by studies at the cellular level (and vice versa)5.
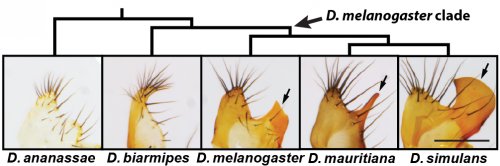
The major discriminating feature of Drosophila melanogaster is a single cell tall!
When first examining the morphogenesis of the posterior lobe, we had no idea where to start. We sought the advice of a colleague across campus, Lance Davidson, who studies the biomechanics of development at the University of Pittsburgh Swanson School of Engineering. Lance was very excited to see our preliminary movies of posterior lobe development in which we monitored apical cell junctions (Movie 1). Even with this new perspective on lobe development, we had no idea exactly what was going on – we could see the apical surfaces of cells changing size and shape as the posterior lobe forms, but of course seeing these changes and knowing what processes cause these alterations are two very different things.
However, apical cell shape changes cover only 2D of the 3D story and examining the entire shape of the cell using single cell fluorescent labeling (Movie 2), indicated that cells of the posterior lobe drastically change their shape along the apical-basal axis, and become extremely tall and thin throughout development, allowing the posterior lobe to project off of the lateral plate. Understanding how this extreme cell shape is controlled and spatially patterned would provide a context in which to connect the activity of gene regulatory networks to the patterning of cellular traits. Initially, we became quite interested in the role that cytoskeletal regulators might play in this process: patterning and stabilizing filaments of both actin and microtubules. However, our investigation uncovered an unexpected perspective on this cellular behavior.
Listen to your collaborators!
One day over our frequent lunchtime conversations, Lance raised the point that the posterior lobe has a cantilevered structure: it leans to one side, and he mentioned that such configuration is rare in epithelial structures. This led him to speculate that maybe we should be looking for some kind of “tether” that would pull the tissue into this arrangement. I remember quite vividly discussing this interesting model with members of the lab, and quickly dismissing it because we had never seen such a tether with our own eyes. Of course, we had only been looking at epithelial and nuclear markers at that point, and so any material that could serve as a tether might go unnoticed if it is translucent and doesn’t express the markers we used. This idea lingered in the back of our minds with little notice before we realized that perhaps Lance was onto something. Performing certain antibody stains, in particular for a septate junction protein Macroglobulin (a kind gift from Dr. Rob Ward, U. Kansas), we noticed that there was a mysterious pattern of background staining in our samples when visualized on the confocal (Figure 2). This caused us to consider the potential role of the apical extracellular matrix in building a posterior lobe.
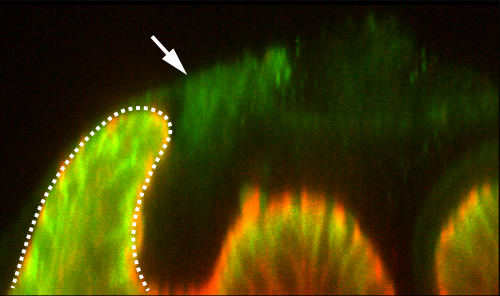
Looking beyond what the eye can see
Although basal extracellular matrices are well known to play critical roles in epithelial sheets6, recent work has shown that apical matrices are also important. Several recent papers had shown that the zona pellucida domain-encoding protein Dumpy is important for shaping the wing by attaching its distal tip to an overlying cuticle7,8. We examined a fluorescently tagged fusion protein for Dumpy, and this revealed a surprisingly intricate web of apical matrix throughout the genitalia that showed prominent connections to the posterior lobe (Movie 3). We developed fluorescent lectin staining protocols to show that this matrix exists in species that lack lobes. However, we found that strong aECM connections to where the lobe would otherwise form are much less pronounced in non-lobed species. Finally, what made this narrative compelling from an evolutionary sense is that RNAi experiments showed that Dumpy expression is required for cells of the lobe to achieve their height. Together, these results demonstrated how making prominent aECM connections is important to the formation of a new structure, and was likely subject to evolutionary changes which alter how the epithelium deposits and interacts with the matrix.
Evolution of novelties: more than meets the eye
What makes this finding surprising is that it reveals the layers of complexity even in such a simple morphological novelty. We had not anticipated that such a complex matrix would exist outside the cells that form a morphologically novel structure. As we generally don’t look to matrices when studying cellular processes, it may be that other epithelial structures have equally elaborate and uncharted apical matrices. More broadly, the story highlights how studying novelties can unveil processes previously unknown and shows how we can zero in on proximal cellular mechanisms that assemble the elaborate structures we see in the multicellular world. Comparing cellular behaviors between species thus offers a unique window into how genetic programs drive physical processes in developing tissues. We suspect that studying the regulatory sequences and networks controlling the expression patterns of Dumpy and other apical ECM components will allow us to go beyond simplistic models of network co-option and the evolution of novelty.
References
- Smith, S. J., Davidson, L. A. & Rebeiz, M. Evolutionary expansion of apical extracellular matrix is required for the elongation of cells in a novel structure. Elife 9, (2020).
- Eberhard, W. G. Sexual selection and animal genitalia. (Harvard University Press, 1985).
- David, J. R., Lemeunier, F., Tsacas, L. & Yassin, A. The Historical Discovery of the Nine Species in the Drosophila melanogaster Species Subgroup. Genetics 177, 1969–1973 (2007).
- Glassford, W. J. et al. Co-option of an Ancestral Hox-Regulated Network Underlies a Recently Evolved Morphological Novelty. Dev. Cell 34, 520–531 (2015).
- Smith, S. J., Rebeiz, M. & Davidson, L. From pattern to process: studies at the interface of gene regulatory networks, morphogenesis, and evolution. Curr. Opin. Genet. Dev. 51, 103–110 (2018).
- Brown, N. H. Extracellular Matrix in Development: Insights from Mechanisms Conserved between Invertebrates and Vertebrates. Cold Spring Harb. Perspect. Biol. 3, a005082–a005082 (2011).
- Ray, R. P. et al. Patterned Anchorage to the Apical Extracellular Matrix Defines Tissue Shape in the Developing Appendages of Drosophila. Dev. Cell 34, 310–322 (2015).
- Etournay, R. et al. Interplay of cell dynamics and epithelial tension during morphogenesis of the Drosophila pupal wing. Elife 4, e07090 (2015).


 (3 votes)
(3 votes)