The people behind the papers: Gabriel Krens and Carl-Philipp Heisenberg
Posted by the Node Interviews, on 25 May 2017
Cell sorting is a critical process during development, as differently specified cells are segregated to the right parts of the embryo. Differences in cell adhesion and cortical tension are thought to be crucial to this process, but the mechanics have been difficult to probe in vivo. This week’s paper, published in the current issue of Development, argues that directed migration – rather than differential tissue surface tension – drives cell sorting during zebrafish gastrulation. We caught up with lead author S.F. Gabriel Krens and his supervisor Carl-Philipp Heisenberg, Professor in the Institute of Science and Technology in Klosterneuburg, Austria.

Carl-Philipp, can give us your scientific biography and the key questions your lab is interested in?
CPH I studied Biology in Munich, did my PhD in the lab of Christiane Nüsslein-Volhard, in Tübingen/Germany and worked as a postdoctoral fellow in the lab of Steve Wilson in London/UK. I started my own lab at the MPI-CBG in Dresden/Germany in 2001 and moved from there to the newly founded IST Austria in Klosterneuburg/Austria a few years ago.
And Gabriel, how did you come to join the Heisenberg lab?
During my PhD I was introduced to work with zebrafish in the research group of Prof. Herman Spaink and my supervisor Dr. Ewa Snaar-Jagalska at the University of Leiden (NL). The focus of my work was more to understand the role of MAPKs in development. Since the importance of these proteins in early embryonic processes, I was also soon exposed to the work of Carl-Philipp and I got very much interested in the multi-disciplinary approach that he was taking.
I met him for the first time in person at ‘the zebrafish conference’ in Dresden, and after a second visit later to the MPI-CBG in Dresden, I knew that this was the place I wanted to go to, to do my postdoctoral research. After finishing my PhD, I joined his lab for my postdoctoral studies, and later also moved with him to I.S.T. Austria.
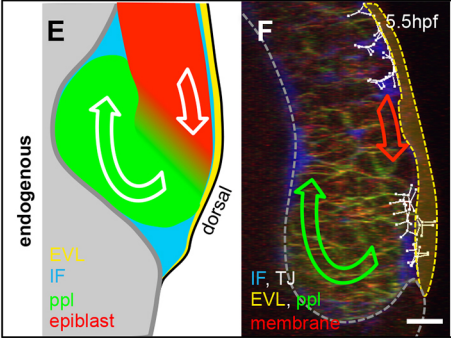
Can you give us the key results of the paper in a paragraph?
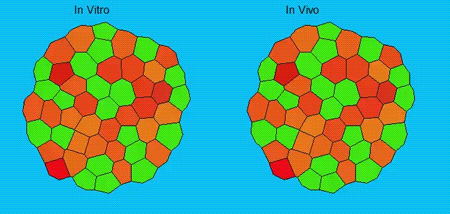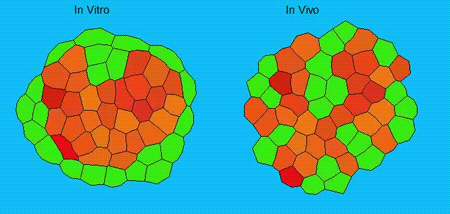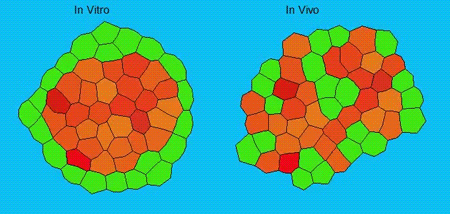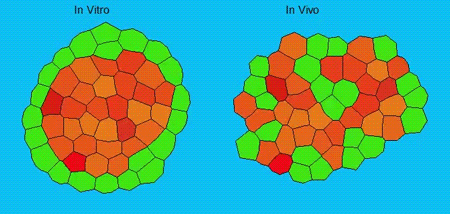
GK One of the key results in my opinion is there was only very little known about the role of the interstitial fluid – in particular in early development. Many interpretations therefore have been drawn on the interaction that different cells display toward each other, without taking into account their direct ‘liquid’ environment of their physiological context: the developing embryo. This notion only appeared to me after performing quite a number of experiments in vitro and trying to understand the differences of germ layer organization between the in vitro data and in vivo gastrulation processes. In addition, I got the chance from Carl-Philipp to visit Wayne Brodland in Waterloo (Canada) and Wayne told me back then that he knew how to extract interfacial tensions from images based on his DITH hypothesis, but that he lacked the experimental data to do so. I had been optimizing the imaging of our cell sorting assay in vitro to the point that we could record on a cell-membrane resolution that would allow us to extract interfacial tensions. After obtaining our first results on experiments performed in culture, I was excited to test the CellFIT-3D in vivo, but we were missing this 3rd ‘liquid’ interface. I noticed that there were gaps between cells in the developing embryo. It did not take us long to consider that these cavities were not empty, but rather fluid-filled (IF), and that we should take this additional fluid interface into consideration too for our tension analysis. Ever since, we have been tightly collaborating with Jim and Wayne to develop the technology, improve image quality and to get to a level of biological understanding of the differences in interfacial tension relationships in vitro and in vivo to the point that we thought was ready to be published.
What made you decide to look at osmolarity in the first place? Is it an underappreciated variable in culture experiments?
GK We already knew for quite some time that the interfacial tension distributions that we found in vitro were not able to explain the behaviour of what we see in vivo. We tried many approached to find out what was different, and one of them was to use the poky mutant fish line from Daniel Wagner. These fish have a defective enveloping layer and are therefore quite susceptible to osmotic variation of the embryo culture medium. By experimenting with this, we discovered that we could actually influence the germ layer organization in these fish by only altering the embryo medium. So far, we were not able to extract tensions in these fish, as the dye to label the IF diffused out at the moment of gastrulation initiation, but this was the main motivation to try to characterise the osmolarity of IF of early zebrafish embryos.
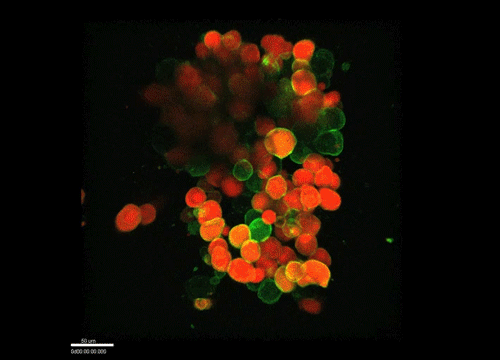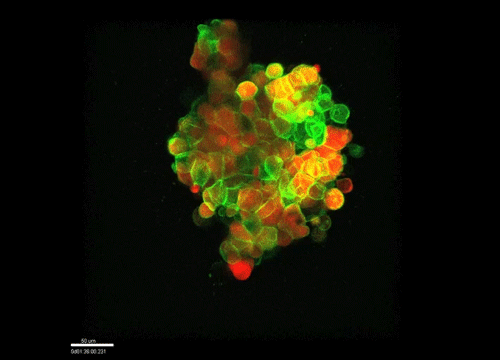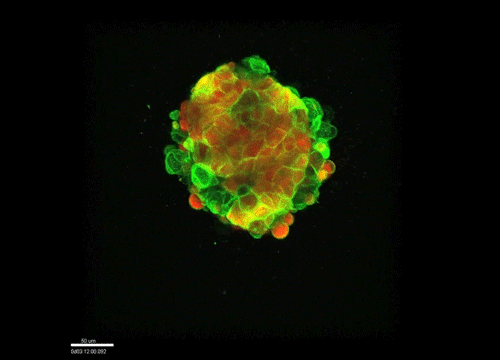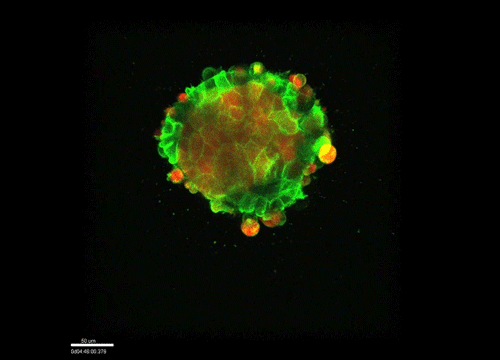
And how do you think osmolarity might influence cell behaviour in your experiments?
GK It is well known that cells respond to osmolarity, and that there is an immediate / short-term response and a long-term response. Changing the osmolarity will change the osmotic pressure and subsequently the hydrostatic pressure in the cells. This hydrostatic pressure needs to be compensated, which can occur by an increase of contractility of the actomyosin cortex and by changing the composition of the cytoplasm / metabolism. On long term – it is likely that also regulators of cytoplasmic composition, such as ion-channels, aquaporins and metabolic regulators, are involved in the process. This would be a nice area to follow up on.
Do you have an idea about the cues that direct the mesendoderm progenitor migration during internalisation?
GK I think that this is an open question in the zebrafish that stands out already for a long time. I find it more and more difficult to believe that we have missed out on that one components in all previous molecular and genetic screens that have been performed so far. On top of that, there as been numerous attempts, and so did we, to find THE cue that directs mesendoderm progenitor cells to the embryonic interior. Therefore, I am starting to believe that this might be more a generic factor, such as the embryonic layout, rather than one single guidance molecule. As we discuss at the end of our manuscript, this could also distribution of IF, as this seems to form gradient at the onset of gastrulation. At this moment this hypothesis goes more toward speculation that anything that is build on data!
When doing the research, was there a particularly exciting result or eureka moment that has stayed with you?
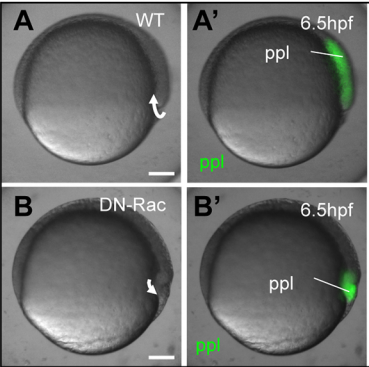
GK During this work, there were quite some eureka results, as we had to overcome a significant amount of technical and intellectual difficulties. But we definitely had a couple of very good moments: The matching simulations output with the CellFIT found to the experiment; the osmolarity rescue experiments in vitro; the DN-Rac data I still find rather stunning as this was an experiment with low hope and a surprisingly awesome result; but most special is the moment that we managed to both extract and measure IF osmolarites, as this is a rather challenging set of experiments that need to come together perfectly to get it to work.
And what about the flipside: any moments of frustration or despair?
GK As mentioned before, we have tried quite some approaches, which all had their challenges. The whole study took pretty long and that means that there were also times that things did not go so smoothly of course and that many things failed or that we got quite some experiments to work with nice supportive data, but that did not make it in the manuscript. We also moved labs in between – which also did not simplify matters – but I am really glad with the final result of the work.
What next for you following this work?
GK Momentarily I am turning my focus to the more technical part of the lab-work that I have been doing. We needed to do quite some ‘expert experiments’, which also demanded quite some manual annotation of image data. Automation of the (image-)analysis of these experiments will be beneficial to a lot of my colleagues. I will also find it really interesting to further get to know if there is a specific component in the IF that might effect cells to have physical properties: what is the composition of IF, how is the composition regulated and I remain curious to find out why mesendodermal germ layer progenitor cells actually end up at the inside of the zebrafish gastrula.
And where will this paper take the Hesienberg lab?
We got particularly interested in understanding how interstitial fluid is accumulating within the early zebrafish blastula, and whether this accumulation is important for cell/tissue morphogenesis and cell fate specification during gastrulation. Our preliminary data clearly support a model where differences in the osmolarity between the inside and outside of the embryo trigger interstitial fluid accumulation and we are currently trying to find out how this affects gastrulation.
Finally – what do you get up to when you are not in the lab?
GK People who know me, know that I do not have to think long to give a reply to this question: I am a passionate mountain biker. The fact that we moved closer to the mountains is a big bonus for someone that come from a country that is know to be extremely flat and below sea level. Besides that, I do some running and drawing.


 (4 votes)
(4 votes)