A Day in the Life of a Hofstenia Lab
Posted by Paul Bump, on 19 September 2024
This article is co-written by Eliza Hirsch and Paul Bump.
Who are we?
Hi, my name is Eliza Hirsch, and I am an undergraduate in the Srivastava Lab at Harvard University. And my name is Paul Bump, and I am a postdoctoral fellow at the Srivastava Lab who works with Eliza. Our lab studies the highly regenerative acoel, Hofstenia miamia. This Hofstenia species originally was collected in Bermuda and now lives in Cambridge, Massachusetts where we study stem cell biology and the evolution of regeneration across animals.
Who is Hofstenia?
Hofstenia miamia is an acoel worm capable of whole-body regeneration, meaning that if you were to bisect one, both halves would regenerate, leaving you with two worms instead of one. Hofstenia maintain a population of adult pluripotent stem cells (aPSCs) that are responsible for regeneration. The regenerative ability of Hofstenia is quite impressive but they are hardly the only systems in which such regenerative ability exists. So why study them? Well, two other key traits of Hofstenia are central to answering this question.
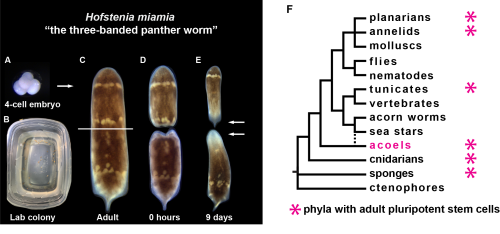
Hofstenia have experimentally accessible embryos, something that is very central to the work we do on the development of the aPSCs. They lay their embryos when they are zygotes, allowing us to study their development from the single cell stage all the way up through hatching and beyond. Hofstenia embryos are also easily accessible due to their abundance. The worms we care for lay zygotes frequently enough and at a high enough volume that we can collect almost every day of the week and on most days, we get a substantial number of them. Thus, as a system Hofstenia allow us to study the development of a highly regenerative organism as well as its adult regenerative ability.
How to work with Hofstenia
To understand what it is like to work daily on Hofstenia we thought it would be best to give a perspective from Eliza who is completing her undergraduate thesis research in the Srivastava Lab:
Hofstenia are relatively easy to care for. In the lab, we keep a large portion of our worm colony in plastic Tupperwares of various sizes with up to sixty worms being stored in any given large Tupperware box. When not being cleaned or collected from or used for any other experimental processes, these boxes sit in incubators. The worms themselves live in artificial sea water that is made in the lab, something which perhaps requires the most time and effort in the caretaking of these animals. Adult worms are fed recently hatched brine shrimp as Hofstenia require live food. Hatchlings and sick worms or worms that may be left unattended over a long weekend without cleaning are fed rotifers as they pose a far smaller risk to the pH of the water and by extension the health of the worms in the event of their death.
To clean the worms and avoid problems with ammonia build up in the water and the like, twice a week we replace the water and wipe down the inside of the container. An important element of worm care is the collection of embryos. The animals lay their embryos on the sides and bottom of the container, and I gently scrape at the walls with a glass pipette to dislodge them and then collect them with the same pipette and deposit them in a dish full of sea water. On top of the wild type worms, the lab also has various lines of transgenic worms that are more sensitive than the wild type worms and much harder to replace and therefore require even more careful care.
Recently the lab has started using a water system for many of the wild type worms that significantly reduces the amount of time spent cleaning the worms as they have circulating water and are no longer in boxes but rather are in tanks. However, since my work depends on very early embryos, my worms are still in boxes so that I have access to those embryos which are more difficult to collect in the water system.

How to crack an egg, starting development in Hofstenia
For my project, I have been collecting embryos every day. Hofstenia embryos are surrounded with a transparent chorion (their eggshell) that must be removed for much of the work that I do with them. Nearly daily I perform a chemical dechorionation on the single-cell zygotes I’ve sorted from the embryos I collected that morning. For this process I use a combination of chemicals and then mouth pipette embryos one by one into twenty-four well plates filled with a combination of artificial sea water and a small amount of antibiotics. Antibiotics are an essential addition as removing the chorion strips the embryos of a key defense against infection. I then raise these one-cell embryos up without a chorion so that I can do experiments at specific developmental stages that would be too sensitive to the dechorionation process.

The future for Hofstenia
Since Hofstenia is still a relatively new research organism, there are many possible questions about them to try to answer in one’s own research. In the future, hopefully many labs will continue to increase our overall understanding of Hofstenia and use that information to evaluate evolutionary and mechanistic questions of how the stem cell lineage develops and allows the regeneration of any missing body part. While one drawback of such a new model system is that many methods for working on the animals have yet to be developed, the future is bright as we will continue developing technologies with which to study Hofstenia development and regeneration.


 (9 votes)
(9 votes)
Dear Eliza,
This is fascinating.
Love,
Donald