BSDB Gurdon Summer Studentship Report (19)
Posted by BSDB, on 1 December 2017
![]() Established by the British Society for Developmental Biology in 2014, The Gurdon/The Company of Biologists Summer Studentship scheme provides financial support to allow highly motivated undergraduate students an opportunity to engage in practical research during their summer vacation. Each year, ten successful applicants spend eight weeks in the research laboratories of their choices, and the feedback we receive is outstanding.
Established by the British Society for Developmental Biology in 2014, The Gurdon/The Company of Biologists Summer Studentship scheme provides financial support to allow highly motivated undergraduate students an opportunity to engage in practical research during their summer vacation. Each year, ten successful applicants spend eight weeks in the research laboratories of their choices, and the feedback we receive is outstanding.
Our eighth report from the 2017 group of student awardees comes from Nicole Serzhantova (student at The University of Edinburgh), who undertook her studentship with Jennifer Nichols at The University of Cambridge.
Understanding the first step in the formation of organismal complexity in the mouse embryo
As biologists, we are very used to poking and prodding very complex systems, be it drosophila or homo sapiens. Rarely is it a prerogative to take a step back and really understand how such complexity arises seemingly out of nowhere. How is that we start off from a single cell and over a period as short as 48 hours (in the mouse at least) we already start seeing complexity arising?
In mice, a single-cell zygote undergoes a series of cleavage cell divisions and morphogenetic changes to form a unipotent epithelial vesicle termed the trophectoderm (TE) enclosing a compact group of pluripotent stem cells known as the inner cell mass (ICM) (Fig.1F).
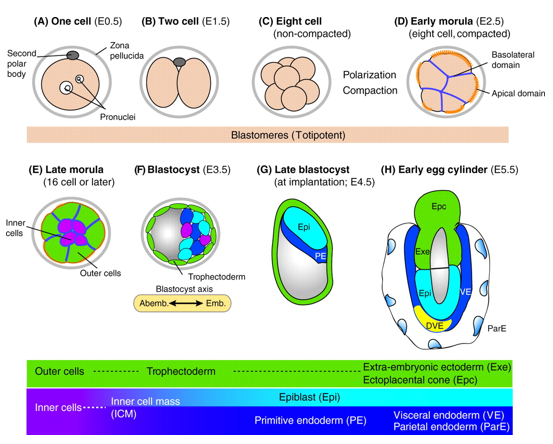
Restriction of cell fate takes place over an extended time period of 48 hours from the 8-cell (morula) stage (Fig.1C), when all cells are indistinguishable from one another and are capable of forming all three germ layers. This feature of cells is termed totipotency and is a transient characteristic of embryonic stem cells before they commit to become either TE or ICM.
At this 8-cell stage compaction of the embryo begins, in which the embryo surface smoothens because of an increase in intercellular adhesion. Cells epithelialise, forming tight junctions (which acts as a seal) between cells, limiting diffusion and what you are left with is fluid-filled epithelial vesicle enclosing a small number of cells (Fig. 1D-F).
It is at point that the first commitment of cells occurs, whereby the outer epithelium (Fig.1E) becomes committed to form the trophectoderm (TE), which subsequently gives rise to the trophoblast layers of the placenta and the trophoblast giant cells. Whilst the small population of enclosed cells commit to becoming the ICM, which is the pluripotent stem cell lineage of the embryo, giving rise to all of the primary germ layers of the foetus and its extraembryonic tissue.
At this stage of development, protein synthesis from maternal mRNA becomes more transient as the embryo’s own gene programs begin to switch on. Once commitment occurs, as the morulae becomes a blastocyst, differential gene expression is seen between the ICM and TE. At day 2 ½ post-fertilisation, all cells within the morula uniformly express most genes, including the pluripotency factor Oct4 and the TE marker Cdx2. Interestingly, at day 3 ½ the first real differential gene expression is observed, where the ICM begins to express Oct4 whilst loosing its ability to express Cdx2 and conversely the trophectoderm begins to express Cdx2 whilst loosing all Oct4 activity. There is a mutual repression by Oct4 and Cdx2 which further consolidates the TE and ICM segregation.
Elucidating the mechanism governing a cell’s ability to become either a unipotent TE cell or a pluripotent stem cell of the ICM can open up new avenues for generating and maintaining pluripotency in vitro. In an attempt to understand this initial lineage segregation, a stem cell line was cultured which removes the Oct4 gene upon treatment with tamoxifen. This cell line also had a florescent tdTomato reporter under the control of a constitutive promoter, meaning that these cells fluoresce red when exposed to light in the blue to green range.
The following cells were cultured in serum-LIF (a differentiation permissive medium) and different portions of the cells were treated with tamoxifen for either 24, 48 or 92 hours to allow for the excision of the Oct4 gene. Once these time points of treatment were reached, cells were microinjected into live mouse morulae and blastocysts. Approximately 5 morulae and blastocysts were microinjected with 8-10 stem cells deficient in Oct4 from each time point as well as un-induced cells form the same batch as a control. These morulae were left for two days to allow for compaction and the first lineage segregation to occur. These were then promptly fixed and immunostained to examine what effect the deletion of Oct4 had on the cell fate choices that the injected stem cells made.
Following what we already know about what happens during development, it can be hypothesized that stem cells that do not have the capacity to make Oct4 will commit to the trophectoderm lineage as they are missing a key pluripotency factor and therefore cannot become pluripotent cells competent of forming all 3 germ layers.
Unfortunately due to rising temperatures in the laboratory during summer (the air conditioning was of course broken on the days the microinjections were performed), upon imaging it was clear that in most cases only 1 stem cell, out of the 8-10 injected, integrated into the embryo. This was prevalent in the un-induced cells also, suggesting that it was not the tamoxifen treatment that caused toxicity to the cells but rather the experimental conditions. Integration rates were higher in morulae than in blastocysts as expected, since by the blastocyst stage cell commitment has already occurred making it harder for integration to ensue.
Out of the cells that did integrate, only 1 appeared to express Cdx2 markers showing that it has adopted a trophectodermal cell fate, whilst the others exhibited a mixture of pluripotency markers. The conclusion from this being that although Oct4 is necessary for cell commitment to the ICM lineage, additional molecular events must underpin this initial commitment in the mouse model. Inferring this from the data is difficult considering how the number of integrated stem cells was so low and future experiments with more tightly controlled conditions are advised.
These results however are concordant with other studies, with Wu and Schöler (2014) reporting that the “establishment of totipotency in maternal Oct4–depleted embryos was not affected, and that these embryos could complete full-term development without any obvious defect.” In addition to this, Wu and Schöler were able to form Oct4 expressing inner cell masses in embryos with complete inactivation of both maternal and zygotic Oct4 expression as well as reprogramming of fibroblasts into fully pluripotent cells by Oct4-deficient oocytes.
This, in conjunction with the results I obtained, tends to indicate that Oct4 is not essential for the initiation of pluripotency but in contrast to its role is critical for the maintenance of pluripotency.
I would like to thank Professor Jennifer Nichols, Ayaka Yanagida, Peter Baillie-Johnson, Thorsten Boroviak, Ken Jones, Tim Lohoff and everyone else at the Cambridge Stem Cell Institute for their invaluable guidance and support throughout my internship. I would also like to thank the British Society for Developmental Biology for granting me the Gurdon Studentship Award without which none of this would have been possible.


 (3 votes)
(3 votes)