BSDB Gurdon/The Company of Biologists Summer Studentship Report #25 – Melissa Gomez
Posted by BSDB, on 18 December 2018
Established by the British Society for Developmental Biology in 2014, The Gurdon/The Company of Biologists Summer Studentship scheme provides financial support to allow highly motivated undergraduate students an opportunity to engage in practical research during their summer vacation. Each year, ten successful applicants spend eight weeks in the research laboratories of their choices, and the feedback we receive is outstanding. You can read accounts from previous years here.
Our fourth report from the 2018 group of student awardees comes from Melissa Gomez (student at The University of Aberdeen) who undertook her research with Martin Collinson (also at Aberdeen).
The Development of microphthalmia in Pax6 mutant mice
Every 4 ½ minutes, a neonate in the USA is born with a birth defect, this totals up to 120,000 babies per year from the USA alone (1). As an embryology student, the underling mechanisms which cause birth defects are of prime interest to me, in the hope that understanding the cause of the defect can potentially lead to prevention. This summer I was able to conduct my own research on a birth defect called microphthalmia.
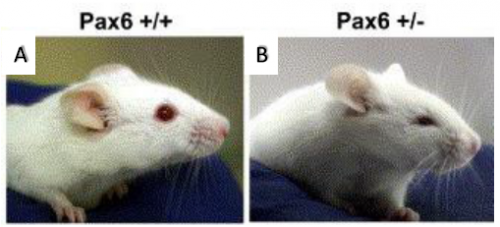
displaying microphthalmia (4).
Microphthalmia is a disorder in which one or both eyes are abnormally small. This birth defect is closely associated with a gene called Pax6, the so-called ‘master regulator gene’ of the eye. Pax6-/- in both mouse and human causes anophthalmia (absence of the eye) due to failure of lens placode induction (2). Pax6+/- causes microphthalmia (Figure 1), as well as aniridia (absence of the iris), cataract and corneal opacity (2). In mice, all Pax6+/- have microphthalmia, but in humans, Pax6+/- individuals usually have aniridia, cataract and corneal problems but tend to have normal size eyes. However, it has been shown that point mutations in Pax6 are strong risk factors in the development of microphthalmia (6)(7). The mechanism for this is not clear, but it can be studied in mice because all Pax6+/- develop microphthalmia. Microphthalmia seems to arise due to the lens being sensitive to Pax6 dosage, for instance it has been found Pax6+/- mice have a 50% reduction in the number of cells in the lens during early embryogenesis (2). The lens is crucial in eye development as it secretes growth factors, thus a reduction in lens cells leads to a decrement in growth factors (3). In addition to this, Pax6+/- lens cells tend to undergo one less round of division when compared to Pax6+/+ mice (2). Why this occurs in Pax6+/- lens remains a mystery, however it can be hypothesised that cell cycle time is increased leading to fewer cell divisions. Thanks to the BSDB Gurdon’s Studentship I was able to further investigate this hypothesis alongside Professor Martin Collinson at the University of Aberdeen.
My projected started with timed matings of Pax6+/- x Pax6+/- mice. At E14.5, the pregnant mice received an injection of iododeoxyuridine (IdU), a thymidine analogue that is incorporated into the replicating DNA of cells in S-phase of the cell cycle. 60 minutes later a second injection of ethynyl-deoxyuridine (EdU), another thymidine analogue, was given. 30 minutes after, mice were killed, and embryos were harvested and fixed in paraformaldehye. The genotypes were confirmed by PCR and electrophoresis using a small piece of tissue from each mouse embryo, Pax6-/- embryos were not used in this experiment because they do not have eyes. After the genotype of each embryo was confirmed, I dehydrated the embryos in ascending concentrations of ethanol (75%, 80%, 95%, 100%) and placed them in xylene. Next, my favourite part of the experiment: paraffin wax embedding. Once the embryos were embedded, a cryotome was used to cut sections which were placed onto poly-L-lysine slides.
Immunohistochemistry was conducted on these slides, Anti- EdU conjugated with a green flurophore (Alexa 488) and Anti-IdU conjugated with a red fluorophore (Alexa 594) were used to detect EdU and IdU labelled cells respectively (Figure 2). DAPI staining was used to visualise all nuclei present in the mouse embryo (Figure 2). The images produced were magnificent, as shown below:
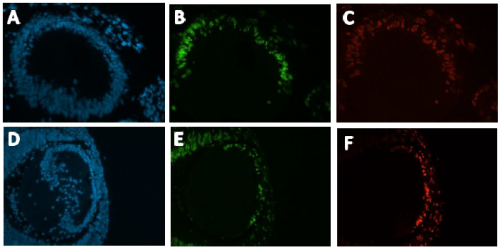
with DAPI. Green corresponds with EdU positive cells. Red corresponds with IdU positive cells. A-C
from a Pax6+/+ developing mouse lens. D-F from a Pax6+/- developing mouse lens.
By counting the proportion of single labelled IdU or EdU cells in the lens epithelium, and the proportion of double labelled cells, the cell cycle time and length of S phase could be calculated using the following equations:
Length of S phase (Ts):
Ts= Ti / (L cells / S cells
Cell Cycle time (Tc):
Tc = Ts / (S cells / P cells)
(Ti = Length of IdU exposure, L cells = IdU only positive cells, S cells = EdU only positive cells, P cells = total number of cells) (5)
On average Ts and Tc lasted longer in Pax6+/+ than Pax6+/- lenses (Figure 3 and 4), however, when a t-test was conducted there was no significant difference shown between Pax6+/+ and Pax6+/- (t- test values; Ts = 0.11947 and Tc = 0.19446). The Pax6+/- lenses were very variable, which perhaps reflects the clinical variability of Pax6 mutation symptoms. If I had extra time, I would repeat the experiment with more embryos to allow for an increased statistical power for Ts and Tc between Pax6+/+ and Pax6+/- mouse embryos.
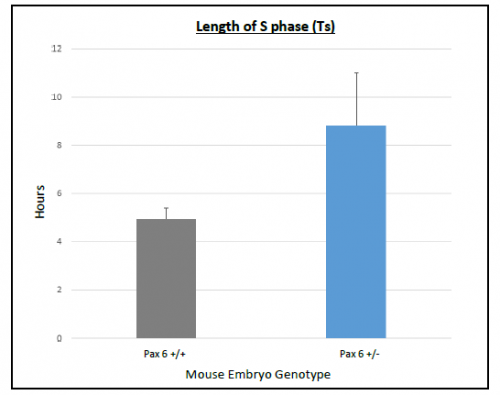
bars representing the standard error of the mean (SEM)s. There was a
trend for S phase to last longer in Pax6+/- compared to Pax6+/+.
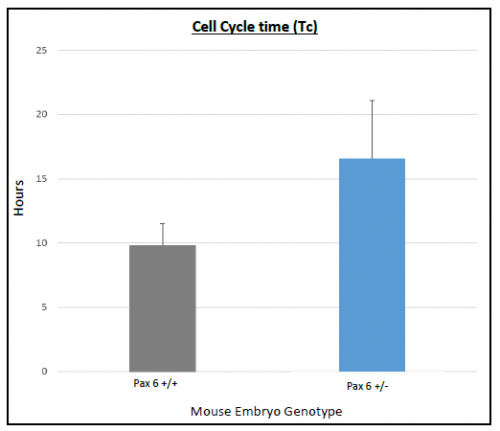
with error bars representing SEM. Cell cycle time lasted longer in
Pax6+/- compared to Pax6+/+
Looking back at my time in the lab, I can’t believe how much I have learned. I could have never imagined myself conducting research independently as an undergrad, however, from week 2 I felt confident enough to proceed with protocols on my own accord. My 8-week project has sadly come to an end, but I feel more excited than ever for a future in developmental research. I would like to thank Professor Martin Collinson and the Gurdon Summer Studentship for giving me this opportunity and making my summer in Aberdeen a lot less grey.




 (5 votes)
(5 votes)