In Development this Week (Vol. 144, Issue 17)
Posted by the Node, on 29 August 2017
Here are the highlights from the current issue of Development:
AdamTS-A keeps the CNS in shape
The Drosophila central nervous system (CNS) is covered by a thick basement membrane that mediates interactions with glial cells and governs the shape of the tissue. Basement membranes are formed from a meshwork of secreted extracellular matrix (ECM) proteins and must be continually remodelled to accommodate growth during development. Metalloproteinases, such as those in the AdamTS family, can break down ECM proteins in the basement membrane and thus allow for tissue expansion. 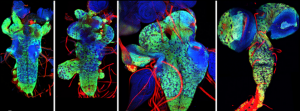 On p. 3102, James Skeath and colleagues identify one protein in the family, AdamTS-A, that is critical for both maintaining the structural integrity of the Drosophila CNS and keeping cell lineages anchored in the tissue. When the function of this protein is reduced, neurons escape the CNS and invade the peripheral tissues of the developing larva. AdamTS-A acts through protease-dependent and possibly protease-independent mechanisms to retain neurons within the developing CNS, and regulates tissue stiffness by restricting Collagen IV/Viking accumulation in the basement membrane. These findings demonstrate the crucial role for extracellular proteases in tissue development and highlight the importance of the basement membrane in shaping the nervous system.
On p. 3102, James Skeath and colleagues identify one protein in the family, AdamTS-A, that is critical for both maintaining the structural integrity of the Drosophila CNS and keeping cell lineages anchored in the tissue. When the function of this protein is reduced, neurons escape the CNS and invade the peripheral tissues of the developing larva. AdamTS-A acts through protease-dependent and possibly protease-independent mechanisms to retain neurons within the developing CNS, and regulates tissue stiffness by restricting Collagen IV/Viking accumulation in the basement membrane. These findings demonstrate the crucial role for extracellular proteases in tissue development and highlight the importance of the basement membrane in shaping the nervous system.
Morphogens and microcolonies: directing stem cell fate
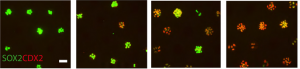 On p. 3042, Aryeh Warmflash and colleagues show that upon stimulation with BMP4, which induces a mixture of fates in large colonies of human embryonic stem cells (hESCs), colonies of just one to eight cells instead acquire trophectoderm-like fate uniformly. They show that above a threshold concentration, BMP4 acts as an ‘on-off’ switch to specify trophectoderm-like cells in these microcolonies, and that a community effect ensures all cells acquire the same fate. In contrast, the mixed cell fates in larger colonies result from the activity of a secondary signal, Nodal, which is produced endogenously by the cells. These findings provide insight into how cells in culture might be directed to specify a single lineage more reproducibly, and demonstrate how the same morphogen signals can be used repeatedly in development to achieve different outcomes, depending on their context.
On p. 3042, Aryeh Warmflash and colleagues show that upon stimulation with BMP4, which induces a mixture of fates in large colonies of human embryonic stem cells (hESCs), colonies of just one to eight cells instead acquire trophectoderm-like fate uniformly. They show that above a threshold concentration, BMP4 acts as an ‘on-off’ switch to specify trophectoderm-like cells in these microcolonies, and that a community effect ensures all cells acquire the same fate. In contrast, the mixed cell fates in larger colonies result from the activity of a secondary signal, Nodal, which is produced endogenously by the cells. These findings provide insight into how cells in culture might be directed to specify a single lineage more reproducibly, and demonstrate how the same morphogen signals can be used repeatedly in development to achieve different outcomes, depending on their context.Pax8+ secretory cells: progenitors of the oviductal epithelium
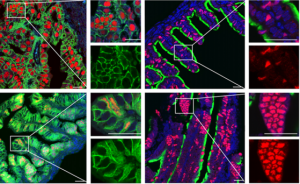 On p. 3031, Pradeep Tanwar and colleagues use lineage tracing in mice to identify the oviductal progenitors as secretory cells expressing the Pax8 marker gene. They show that these cells actively divide in the oviductal epithelium, and that this cell population is expanded in humans who are predisposed to ovarian cancer. Like progenitor populations in other tissues, the Pax8+ cells respond to canonical Wnt signalling, which governs their differentiation into ciliated cells and simultaneously maintains the stem cell-like population. These results are a step forward in understanding tissue homeostasis in the oviduct, and may provide insight into how cell proliferation is regulated and subsequently becomes dysregulated in ovarian cancer.
On p. 3031, Pradeep Tanwar and colleagues use lineage tracing in mice to identify the oviductal progenitors as secretory cells expressing the Pax8 marker gene. They show that these cells actively divide in the oviductal epithelium, and that this cell population is expanded in humans who are predisposed to ovarian cancer. Like progenitor populations in other tissues, the Pax8+ cells respond to canonical Wnt signalling, which governs their differentiation into ciliated cells and simultaneously maintains the stem cell-like population. These results are a step forward in understanding tissue homeostasis in the oviduct, and may provide insight into how cell proliferation is regulated and subsequently becomes dysregulated in ovarian cancer.PLUS…
Meeting Review
Reviews


 (No Ratings Yet)
(No Ratings Yet)