The forces that shape us: Mechanics of mammalian neural tube morphogenesis
Posted by Gabriel Galea, on 7 July 2017
Introduction to the biomechanics of neurulation
Those of us who go to the gym are accustomed to thinking of mechanical forces shaping our bodies. Physiological (e.g. determination of bone mass and architecture), pathological (e.g. aneurysm rupture) and even socio-cultural (e.g. lip plates of the Mursi tribe) examples come to mind. The form of most of our organs is templated during embryonic morphogenesis, during which the embryo folds and contorts itself into complex shapes. Origami-like folding of the neuroepithelium to form the neural tube, the embryonic precursor of the central nervous system, has long served as a paradigm of morphogenesis. This process of primary neurulation is fundamentally biomechanical; in mammals it requires progressive tissue-level shape changes to proceed. Failure of these processes, such as insufficient folding of the neuroepithelium at “hinge points”1, are associated with failure of neural tube closure resulting in neural tube defects (NTDs) such as spina bifida. Determining the biomechanics of neural tube closure is therefore a key aim both to understand this fundamental morphogenetic process, and identify causes of NTDs. Here we describe how we have begun to tease apart the biomechanics of neural tube closure in the mammalian spinal region, as recently reported2.
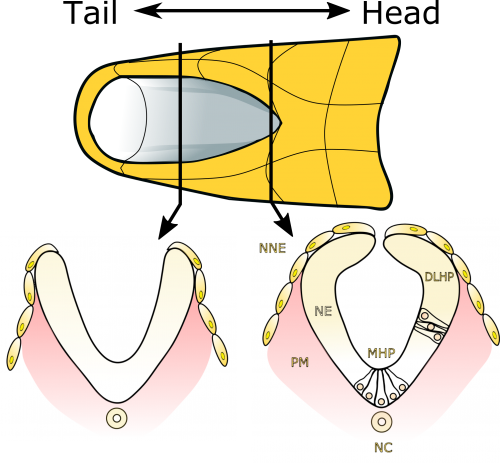
Neural tube closure is a multistep process that requires the coordinated activity of cells of different origins3. It starts with bending of the flat neural plate, creating two opposing neural folds, which progressively elevate and fuse (Figure 1). The fusion process requires the formation of cellular protrusions by non-neural ectoderm cells at the neural fold tips4. These protrusions reach across the midline and “zipper” down the length of the embryo. Zippering reduces the length of the open region of the neural plate, referred to as the posterior neuropore (PNP, Figure 2), as development progresses. PNP length is therefore commonly measured as a readout of the progression of neurulation, and differences in PNP length are extremely useful in identifying developmental stages at which neurulation becomes disrupted. However, in our recent paper we focused on the “perpendicular” process of neural fold apposition, which narrows the PNP. Progressive PNP narrowing also occurs as development progresses, but it has been less intensively studied in recent years.
Whereas significant progress has been made in characterizing the molecular and cellular mechanisms underlying primary neurulation, how these mechanisms biomechanically orchestrate tissue-level shape change is still poorly understood. In our recent review on the topic3 we emphasized that most of the previous work on neurulation biomechanics has been carried out in lower vertebrates and less is known about mammalian neurulation. Lessons learned in simpler models, such as Xenopus5,6 and Ciona7, have substantially advanced our understanding of neurulation as an evolutionarily conserved process. However, extrapolating mechanisms from these models to higher vertebrates must be done with caution given marked differences in the structure of their neural plate and timing over which neurulation occurs3. Thus, our recent paper builds on findings from these simpler models and provides a uniquely mammalian view of the mechanics of primary neurulation.
How the study developed and evolved
Our study started several years ago when Professor Andrew Copp used a glass needle to incise the zippering point in a mouse embryo so as to generate a mechanical model of spina bifida. Unexpectedly, he observed that zippering point incisions caused the neural folds to instantly flip apart, suggesting tension within the surrounding tissue had been pulling them into a more lateral position. This intriguing observation in itself suggested that neural tube closure does not progress through the medial convergence of lateral tissues pushing the folds together as, if that had been the case, incising the zippering point would not have resulted in lateral displacement of the neural folds. Dr Young June Cho, the paper’s second author, bravely took on this finding during his PhD, confirming and expanding the initial observation. However, this model was limited by having to physically incise the neural tube, which carries the potential of inadvertently moving the neural folds. Although physical approaches to testing biomechanics, such as measuring expansion of microsurgical slits8, have been used extensively, we wanted to move towards using less physically invasive methods, namely laser ablation. This became possible in part thanks to the acquisition of a new multiphoton microscope with a Mai Tai laser so powerful it can (accidentally!) set fire to paper, and in part thanks to optimisation of live-embryo positioning and manipulation methods. Laser ablations allowed us to ablate long (>300 µm) lines of tissue from the zippering point along the embryo’s dorsal midline.
This laser ablation method used throughout the paper was set up by the study’s first author, Dr Gabriel Galea, who joined the group on a Wellcome Trust postdoctoral fellowship specifically designed to work on this project. Gabriel had previously collaborated with Prof Copp’s group during his PhD, which was on skeletal cellular mechanobiology. Although the change of fields from bone to neural tube biology was not easy, being a veterinarian he has ample practice in applying knowledge from one situation to another (cats are not small dogs and ferrets are not small cats, but the considerations for treating flea infestations are similar in all three!). The importance of biomechanics to the skeleton is well established: mechanical loading is accepted to be the primary functional determinant of bone mass and architecture. This dogma was established in part thanks to pioneering studies by Gabriel’s PhD supervisors (also vets!), Professors Jo Price and Lance Lanyon. Lance had, many years ago, used strain gauge chips intended for engineering purposes to measure mechanical strains (defined as the percentage change in dimension) in bone. Unfortunately, even the smallest strain gauges are stiffer than neurulation stage embryos, so a non-invasive method was needed to map strains associated with zippering point ablation. Again, engineering provided a solution, in the form of digital image correlation analysis, wherein the relative displacement of pre-placed dots on an object’s surface are used to calculate strains. However implementation of this approach has limitations when applied to biological tissues. Here we turned to a hobby programmer (who also happens to be Gabriel’s father) to script the code for a “Biological Deformation and Strain Measurement” program, quickly renamed “Tissue Deformation and Strain Measurement” (TDSM, which will be available here https://github.com/gauden/tdsm).
After initial validation and testing on simulated datasets, TDSM worked seamlessly when applied to confocal images of mouse PNPs live-imaged before and after zippering point ablation to generate area strain maps. However, the patterns of area strain it showed were so perplexing we described them as “preliminary” even though analyses of sequential embryos all showed the same thing. First of all, they showed long-ranging tissue deformation following laser ablation, far beyond the zippering point, which was unexpected. Secondly, they showed that the tissue around the zippering point itself underwent expansion following ablation. We had confidently assumed the zippering point would be the force-generating structure pulling the neural folds towards the midline, but if that had been the case why would the tissue around it expand following its ablation? Rather, we had expected this region of tissue to retract to a smaller, un-stretched state. Instead we observed tissue constriction far caudally, in the open region of the PNP.
Constriction of the open region suggested a tissue-level mechanism by which the neural folds could be pulled towards the midline. We wanted to see if this tissue constricted during ongoing neurulation but, although we can culture embryos in roller bottle culture for at least 48 hrs, this system precludes visualisation. We therefore reluctantly tried live embryo culture under static conditions while imaging by confocal microscopy, during which the embryos retained a strong heart beat and clear yolk sac circulation. In our setup, even over a relatively short time period (~2 hrs) the embryos underwent substantial morphogenetic movement with very significant narrowing of the posterior neuropore (Video 1, as shown in Galea et al.2). As this happened, the apical surface area of cells in the open region on average decreased, suggesting apical constriction was ongoing. Actomyosin-driven apical constriction is an evolutionarily conserved force-generating mechanism9 and actomyosin is apically enriched in cells of the open neuropore10.
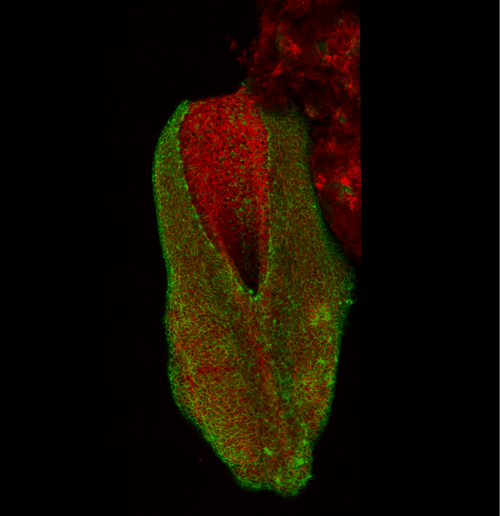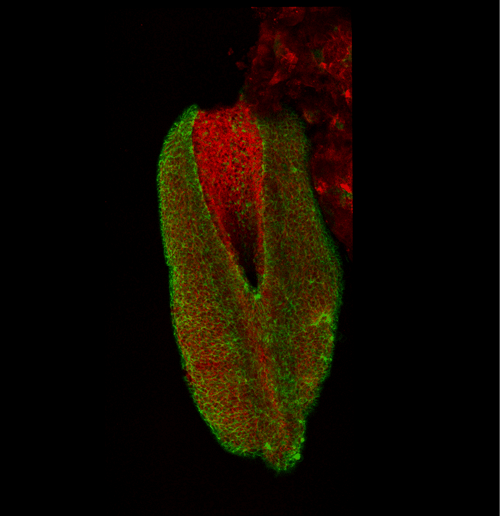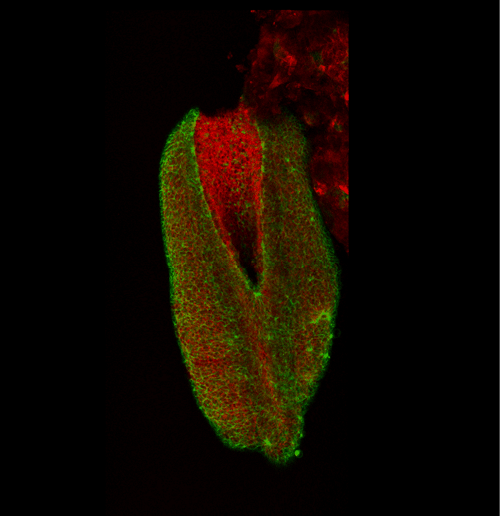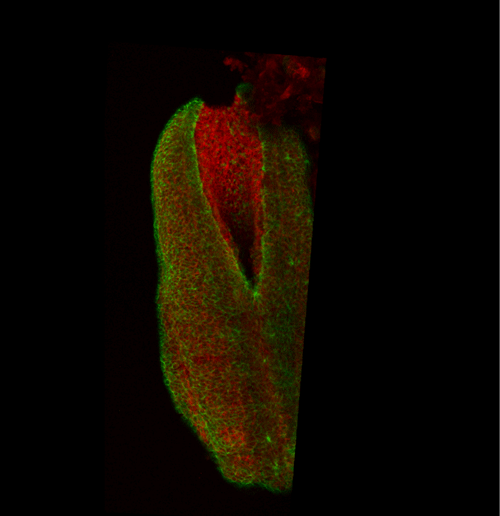
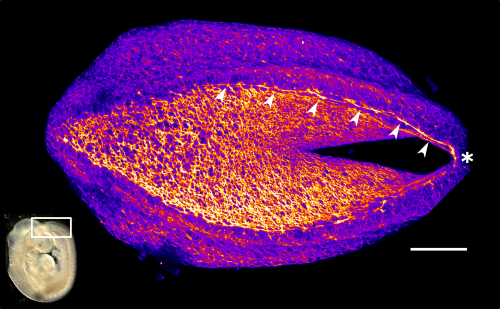
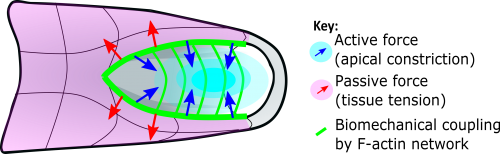
This apical actomyosin enrichment had previously been shown in cryostat sections. However, we wanted to visualise it in all its full three-dimensional glory to observe its integration and extent to the zippering point. In so doing we observed that F-actin formed a discrete, continuous cable reaching all the way from the constricting open zone, along the neural folds, up to the zippering point (Figure 2). Laser ablation of the tissue through which this cable runs also results in the neural folds flipping apart, suggesting that the cable biomechanically couples the neuropore. Taking all these findings together produces our working model that the main forces driving neural tube closure are not medial convergence of the surrounding tissue, and not some sort of “pulling” force at the zippering point, but rather constriction of the open neuropore region (Figure 3). The forces generated here are then transmitted to the zippering point by the coupling F-actin cable.
Submission and review process
This demonstration of long-ranging biomechanical coupling in a mammalian embryo by an F-actin cable is the main ‘take home message’ of our story, although we do also present further findings in the paper. Given the clinical importance of neural tube closure, we initially submitted our paper to a clinically-focused high impact journal, but were told that although the “editors recognized that [our] developmental biology studies were very well done… these findings would be better suited for a developmental biology-focused venue.” Undeterred, and convinced our findings had broad appeal to a wide readership, we submitted our manuscript to PNAS. Here it received positive reviews which largely requested rewriting sections to make them more accessible. It was actually quite a pleasant experience to feel that addressing reviewers’ comments was improving our manuscript; amending one or two conclusions felt not to be sufficiently robust while generally improving clarity and focus. We particularly appreciated the recognition that our field is still at “the dawn of biomechanics in the early mouse embryo.”
Future outlook
Our ultimate aim in delineating the biomechanics of morphogenesis is to identify and prevent causes of their failure which lead to congenital defects, or to stage-specifically bolster mechanisms which may be deficient in pathological states. In the case of the neural tube, the 3D biomechanical analyses we undertook have fundamentally changed how we think of the progression and completion of spinal neurulation, raising new hypotheses for why they may fail leading to spina bifida. Our own analyses are now expanding beyond the specific roles of the zippering point and adjacent neural folds, to tissues to which they are mechanically coupled several hundred microns away. This new focus is helping us make new cell level observations relevant to the development of spina bifida as well as providing new insights into the integration of signalling cascades and environmental/teratogenic stimuli by biomechanical requirements. Ultimately, as with any research, each question we have answered has raised a dozen more, so watch this space!
Gabriel L Galea and Evanthia Nikolopoulou
UCL Great Ormond Street Institute of Child Health, London, UK
References
1 Ybot-Gonzalez, P. et al. Neural plate morphogenesis during mouse neurulation is regulated by antagonism of Bmp signalling. Development 134, 3203-3211, doi:10.1242/dev.008177 (2007).
2 Galea, G. L. et al. Biomechanical coupling facilitates spinal neural tube closure in mouse embryos. Proceedings of the National Academy of Sciences of the United States of America 114, E5177-E5186, doi:10.1073/pnas.1700934114 (2017).
3 Nikolopoulou, E., Galea, G. L., Rolo, A., Greene, N. D. & Copp, A. J. Neural tube closure: cellular, molecular and biomechanical mechanisms. Development 144, 552-566, doi:10.1242/dev.145904 (2017).
4 Rolo, A. et al. Regulation of cell protrusions by small GTPases during fusion of the neural folds. eLife 5, e13273, doi:10.7554/eLife.13273 (2016).
5 Sokol, S. Y. Mechanotransduction During Vertebrate Neurulation. Current topics in developmental biology 117, 359-376, doi:10.1016/bs.ctdb.2015.11.036 (2016).
6 Vijayraghavan, D. S. & Davidson, L. A. Mechanics of neurulation: From classical to current perspectives on the physical mechanics that shape, fold, and form the neural tube. Birth defects research 109, 153-168, doi:10.1002/bdra.23557 (2017).
7 Hashimoto, H., Robin, F. B., Sherrard, K. M. & Munro, E. M. Sequential contraction and exchange of apical junctions drives zippering and neural tube closure in a simple chordate. Developmental cell 32, 241-255, doi:10.1016/j.devcel.2014.12.017 (2015).
8 Benko, R. & Brodland, G. W. Measurement of in vivo stress resultants in neurulation-stage amphibian embryos. Annals of biomedical engineering 35, 672-681, doi:10.1007/s10439-006-9250-1 (2007).
9 Murrell, M., Oakes, P. W., Lenz, M. & Gardel, M. L. Forcing cells into shape: the mechanics of actomyosin contractility. Nature reviews. Molecular cell biology 16, 486-498, doi:10.1038/nrm4012 (2015).
10 Escuin, S. et al. Rho-kinase-dependent actin turnover and actomyosin disassembly are necessary for mouse spinal neural tube closure. Journal of cell science 128, 2468-2481, doi:10.1242/jcs.164574 (2015).


 (2 votes)
(2 votes)