Full-term development of quail chick by ICSI
Posted by Mizushima S, on 18 October 2014
The eggs of domestic birds have been used in the study of developmental biology, leading to the extensive accumulation of knowledge on embryonic development. However, the early events involved in bird development, particularly the mechanism underlying fertilization, have not been elucidated in as much detail as those of other species of animals. The ooplasm in avian eggs is opaque, which makes it difficult to manipulate in vitro for artificial fertilization systems such as intracytoplasmic sperm injection (ICSI). Furthermore, a hen must be sacrificed to obtain a single egg due to the difficulties associated with the constant induction of multiple ovulations.
In our previous study published in Biology of Reproduction (69:1651-1657, 2003), ‘‘Fertilization and development of quail oocytes after intracytoplasmic sperm’’, we successfully established the first avian ICSI system; however, live chicks had never been produced until recently. Although fertilization in birds is polyspermic, namely the entry of many sperm (approximately 60 in chickens) is permitted but only one sperm nucleus participates in zygotic formation with the female pronucleus, the single ejaculated sperm of a quail or chicken was capable of activating the egg for the fertilization process and subsequent blastoderm development 24 hr after ICSI. However, the rate at which the blastoderm developed (16%) was limited, and did not go beyond blastoderm stage X even when the period of cultivation of quail embryos was extended to 72 hr after ICSI. We could not determine why fertility was low even though a single chicken or quail sperm was able to effectively activate the mouse egg. I subsequently joined Shizuoka University in 2011, and the research performed there revealed that the rate of pronuclear formation in quail ICSI-eggs was very low, and also that first cleavage of the ICSI-egg took 1.5 hours longer than that with in vivo fertilization. However, the rate of pronuclear formation increased when the egg was injected with a single sperm in combination with calcium chloride or strontium chloride. These findings prompted us to speculate that a single quail sperm may not be sufficient to induce increases in Ca2+ concentrations in the egg cytoplasm for the pronuclear formation of quail eggs. Polyspermy may play a pivotal role as a Ca2+source in egg activation.
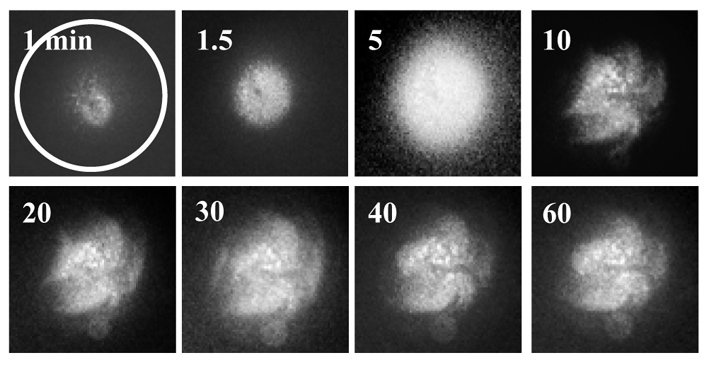
I was fortunate to meet with Drs. Inaba and Shiba in the University of Tsukuba, who are experts on Ca2+ imaging analysis, and collaborated with them to image increases in intracellular Ca2+ concentrations in the quail ooplasm. At that time, I successfully produced the first live quail chick by ICSI with sperm extracts (SE). This baby was named ‘‘Megumi’’, which means “The grace of God” in Japanese. The delayed cleavage of ICSI-eggs was prevented by microinjecting 2 ng SE (equivalent to 200 sperm) and the embryo underwent normal development beyond stage X at a high rate. The microinjection of 2 ng SE into quail egg evoked two phases of Ca2+ changes; multiple, long-lasting spiral-like Ca2+ waves that followed an initial transient increase in Ca2+ concentrations (See Fig), which have never been demonstrated in other species. By the end of 2012, I had finished a pilot study involving biochemical analyses to identify the sperm-derived egg-activating factors (sperm factors) that triggered these spiral-like Ca2+ oscillations. Phospholipase Czeta (PLCZ) was easily determined to be an inducer of transient increases in Ca2+ concentrations. However, difficulties were associated with identifying the sperm factors that induced spiral-like Ca2+ oscillations because a single microinjection of each candidate factor failed to evoke these oscillations. We unintentionally discovered that a simultaneous injection of aconitate hydratase (AH) and citrate synthase (CS) induced spiral-like Ca2+ oscillations, similar to those caused by the injection of SE.
Megumi and her offspring
Regarding the functions of the two characteristic increases in Ca2+ concentrations in quail fertilization, PLCZ-induced transient increases in Ca2+ concentrations were found to be necessary for the resumption of meiosis, similar to that in mammals, whereas spiral-like Ca2+ oscillations alone did not complete the fertilization process. However, the microinjection of neither PLCZ cRNA alone nor AH and CS cRNAs induced the normal development of ICSI-derived zygotes, whereas the simultaneous injections of these 3 factors enabled them to hatch. Therefore, AH and CS-generated Ca2+ signaling suggests a novel role for fertilization cellular event independent of PLCZ-induced Ca2+ signaling in birds. Spiral-like Ca2+ oscillations may function as the major driving force for cell cycle progression in early embryos. However, it has not yet been determined how they enhance the development of the early embryo.
It is our hope that the successful production of healthy chicks after ICSI will lead to significant advances in the introduction of genes into birds as well as cloning technology. ICSI–mediated gene transfer would not only streamline the procedure, but also overcome the low efficiency of avian transgenesis by conventional techniques such as the production of germline chimera. In addition, the development of somatic cell nuclear transfer techniques, in combination with 3 sperm factors, should contribute to the protection of endangered species as well as restoration of extinct species.
Mizushima, S., Hiyama, G., Shiba, K., Inaba, K., Dohra, H., Ono, T., Shimada, K., & Sasanami, T. (2014). The birth of quail chicks after intracytoplasmic sperm injection Development, 141 (19), 3799-3806 DOI: 10.1242/dev.111765



 (6 votes)
(6 votes)