In Development this week (Vol. 138, Issue 11)
Posted by Seema Grewal, on 10 May 2011
Here are the highlights from the current issue of Development:
Dronc regulates Numb and neuroblast formation
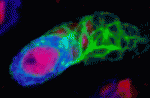
The ability of stem cells to maintain a balance between self-renewal and differentiation is crucial for development and tissue homeostasis. In Drosophila neuroblasts, the tumour suppressor Numb restricts proliferation and self-renewal in differentiating daughter cells but how its activity is regulated is unclear. Here, Bingwei Lu and colleagues reveal a novel mechanism for the control of Numb activity (see p. 2185). They show that phosphorylation of Numb at conserved sites modulates its tumour suppressor activity and that the antagonist actions of Polo kinase and protein phosphatase 2A control Numb phosphorylation. Expression of Numb-TS4D (a phosphomimetic form of Numb) abolishes Numb activity, they report, and leads to the formation of ectopic neuroblasts. They also identify the Dronc caspase as a Numb binding partner and show that Dronc overexpression suppresses the effects of Numb-TS4D in an apoptosis-independent and possibly non-catalytic manner. By contrast, reduction of Dronc activity enhances phospho-Numb-induced ectopic neuroblast formation. Together, these results provide new insights into neural stem cell homeostasis in Drosophila.
Stem cell development goes live

Stem cells are maintained by signals from their local microenvironment but it has been hard to study exactly how stem cell behaviour is controlled. Now, Lucy Morris and Allan Spradling describe a culture method for live imaging Drosophila ovarian development within the germarium and use it to test some long-held beliefs about ovarian follicle development (see p. 2207). The germarium is a structure at the anterior tip of ovarioles that produces new ovarian follicles by controlling follicle and germline stem cell (GSC) division and nurturing their developing daughters. The researchers confirm, for example, that GSC divisions are oriented with respect to the germarium’s anteroposterior axis. They also show that somatic escort cells (the glial-like partners of early germ cells) do not adhere to and migrate with GSC daughters as previously proposed, but pass the GSC daughters from one escort cell to the next using dynamic membrane activity. These and other results establish the live imaging system as a valuable tool for the study of stem cell biology.
p45NF-E2 controls intrauterine growth
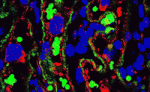
In mice, the absence of the leucine zipper transcription factor p45NF-E2 results in thrombocytopenia (reduced platelet numbers), impaired placental vascularisation and intrauterine growth restriction (IUGR). It is generally assumed that the lack of embryonic platelets causes the growth problems seen in p45NF-E2-deficient embryos, but now, Berend Isermann and colleagues report that the placental defect and IUGR of p45NF-E2 null mouse embryos is unrelated to thrombocytopenia (see p. 2235). Instead, they show that p45NF-E2 is expressed in trophoblast cells where it is required for normal syncytiotrophoblast formation, placental vascularisation and embryonic growth. Expression of p45NF-E2 in labyrinthine trophoblast cells, they report, colocalises with the expression of Gcm1, a zinc-finger transcription factor crucial for syncytiotrophoblast formation. Finally, they show that p45NF-E2 cell-autonomously represses Gcm1-dependent syncytiotrophoblast formation by inhibiting acetylation in vitro and in vivo. The identification of this novel function for p45NF-E2 during placental development provides new insights into the mechanisms underlying IUGR, a poorly understood but common complication of human pregnancies.
miR165: a plant dose-dependent positional cue
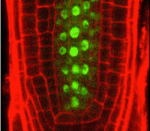
Cell fate determination by positional cues occurs during both plant and animal development. Although some positional cues have dose-dependent effects in animals, this type of cue has not been identified in plants. Here, however, Keiji Nakajima and colleagues show that microRNA165 (miR165) non-cell-autonomously regulates the differentiation of multiple cell types in the Arabidopsis root in a dose-dependent manner (see p. 2303). The Arabidopsis root consists of a central stele (which contains the pericycle layer and the xylem) surrounded by layers of endodermis, cortex and epidermis. Endodermis-derived miR165/166 is known to specify xylem differentiation in the root meristem by suppressing the expression of the transcription factor PHABULOSA (PHB) in the stele. Using an inducible miR165 expression line, the researchers now reveal that endodermis-derived miR165 acts in a dose-dependent manner to establish a PHB expression gradient across the stele that controls the differentiation of two xylem cell types and the pericycle. Thus, these studies reveal that plant development requires at least one dose-dependent positional cue.
Extracellular Engrailed signals get direct
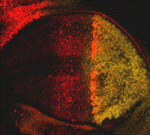
Although homeoprotein transcription factors are best known as cell-autonomous developmental regulators, several homeoproteins have direct non-cell-autonomous activities in the developing vertebrate nervous system. But do homeoproteins also act as signalling molecules during invertebrate development? On p. 2315, Alain Joliot, Florence Maschat and co-workers present the first in vivo evidence for homeoprotein signalling in Drosophila. They use detergent-free immunostaining to reveal an extracellular pool of the homeoprotein Engrailed (En) in the fly wing disc. They then use a secreted single-chain anti-En antibody to show that En is a short-range signalling molecule that participates in the development of the wing’s anterior crossvein. Finally, they report that, in contrast to the repressive effect of En on decapentaplegic (dpp) expression, where it acts intracellularly as a transcription factor, extracellular En activity helps to form the anterior crossvein by enhancing Dpp signalling. The researchers propose, therefore, that direct signalling by homeoproteins is an evolutionarily conserved mechanism that is involved in the development of multiple tissue types.
Blood vessels guide 3D lung branching
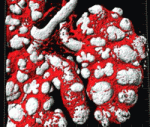
Traditionally, blood vessels are regarded as an inert network of tubes that supply tissues with nutrients and oxygen, but recent studies suggest that blood vessels play perfusion-independent roles in early development. Now, on p. 2359, Eli Keshet and co-workers report that blood vessels also determine the reproducible branch pattern of lung airways in mice. During lung development, the coordinated branching of epithelial and vascular tubes culminates in their co-alignment in the mature organ. By ablating the lung vasculature in vivo and in lung explants, the researchers show that, although the first two-dimensional round of epithelial branching proceeds at a nearly normal rate, branching events that require rotation to change the branching plane into the third dimension are selectively affected. This role of the vasculature is independent of perfusion, flow or blood-borne substances but can be partly explained by perturbation of the expression of stereospecific branching regulators such as FGF10. Together, these results reveal a novel perfusion-independent role for the vasculature in directing three-dimensional organogenes.


 (No Ratings Yet)
(No Ratings Yet)