In Development this week (Vol. 140, Issue 8)
Posted by Seema Grewal, on 26 March 2013
Here are the highlights from the new issue of Development:
Molecular map of posterior hypothalamus
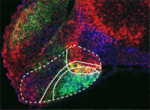 The hypothalamus is a key integrative centre in the vertebrate brain that regulates many essential functions, including homeostasis and stress responses. Several transcription factors that are essential for hypothalamic development have been identified but the production of diverse neuron types in this complex brain region is poorly understood. Here (p. 1762), Andrea Wolf and Soojin Ryu identify the transcription factors required for the specification of two distinct neuron types in the zebrafish posterior hypothalamus. They show that the transcription factor Fezf2 is important for the early development of the posterior hypothalamus. Furthermore, the differential expression of Fezf2, Otp, Foxb1.2 and Sim1a defines distinct subdomains in the posterior hypothalamus during neuronal specification. The neuron types that produce the hypothalamic hormones Vasoactive intestinal peptide (Vip) and Urotensin 1 (Uts1) develop in these different subdomains, they report, and Vip neuron specification requires Otp and Sim1a whereas Uts1 neuron specification requires Fezf2, Sim1a and Foxb1.2. Together, these findings provide mechanistic insights into the generation of neuronal diversity in the hypothalamus.
The hypothalamus is a key integrative centre in the vertebrate brain that regulates many essential functions, including homeostasis and stress responses. Several transcription factors that are essential for hypothalamic development have been identified but the production of diverse neuron types in this complex brain region is poorly understood. Here (p. 1762), Andrea Wolf and Soojin Ryu identify the transcription factors required for the specification of two distinct neuron types in the zebrafish posterior hypothalamus. They show that the transcription factor Fezf2 is important for the early development of the posterior hypothalamus. Furthermore, the differential expression of Fezf2, Otp, Foxb1.2 and Sim1a defines distinct subdomains in the posterior hypothalamus during neuronal specification. The neuron types that produce the hypothalamic hormones Vasoactive intestinal peptide (Vip) and Urotensin 1 (Uts1) develop in these different subdomains, they report, and Vip neuron specification requires Otp and Sim1a whereas Uts1 neuron specification requires Fezf2, Sim1a and Foxb1.2. Together, these findings provide mechanistic insights into the generation of neuronal diversity in the hypothalamus.
Afadin: making and shaping tubules
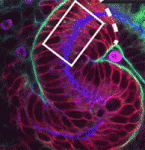 The formation and elongation of polarised epithelial tubules is essential for the structure and function of several metazoan organ systems but the molecular mechanisms that regulate tubulogenesis are largely unknown. Here (p. 1774), Denise Marciano and colleagues provide new insights into tubulogenesis by studying the developing mouse nephron. The researchers show that kidney mesenchymal cells contain Par3-expressing microdomains on adjacent cells. These microdomains coalesce to form a continuous lumen, which elongates by extension and by additional de novo lumen formation. Both lumen formation and elongation require afadin, a nectin adaptor protein that is implicated in adherens and tight junction formation. Using mice that lack afadin in nephron precursors, the researchers demonstrate that afadin is required for the coalescence of Par3-expressing microdomains, which is needed to establish apical-basal polarity and generate a continuous lumen. These results reveal a novel mechanism for lumen formation and morphogenesis in vivo in which afadin plays a central role through its recruitment of polarity and junctional proteins to sites of lumen formation.
The formation and elongation of polarised epithelial tubules is essential for the structure and function of several metazoan organ systems but the molecular mechanisms that regulate tubulogenesis are largely unknown. Here (p. 1774), Denise Marciano and colleagues provide new insights into tubulogenesis by studying the developing mouse nephron. The researchers show that kidney mesenchymal cells contain Par3-expressing microdomains on adjacent cells. These microdomains coalesce to form a continuous lumen, which elongates by extension and by additional de novo lumen formation. Both lumen formation and elongation require afadin, a nectin adaptor protein that is implicated in adherens and tight junction formation. Using mice that lack afadin in nephron precursors, the researchers demonstrate that afadin is required for the coalescence of Par3-expressing microdomains, which is needed to establish apical-basal polarity and generate a continuous lumen. These results reveal a novel mechanism for lumen formation and morphogenesis in vivo in which afadin plays a central role through its recruitment of polarity and junctional proteins to sites of lumen formation.
Ubiquitylation promotes planar polarity
 Planar cell polarity depends on the asymmetric localisation of core planar polarity proteins at apicolateral junctions. This asymmetric distribution probably develops through amplification of an initial asymmetry and seems to require the regulation of core protein levels. Now, Helen Strutt, Elizabeth Searle and co-workers (p. 1693) show that two distinct ubiquitylation pathways regulate the junctional levels and asymmetry of core planar polarity proteins in Drosophila. The researchers report that a Cullin-3-Diablo/Kelch ubiquitin ligase complex and the deubiquitylating enzyme Fat facets regulate the levels of the core planar polarity proteins Dishevelled and Flamingo, respectively, at apicolateral junctions but have no effect on the total cellular levels of Dishevelled and Flamingo. Notably, both increases and decreases in the junctional levels of core proteins caused by disruption of the ubiquitylation machinery reduce core protein asymmetry and disrupt planar cell polarity. Thus, the researchers suggest, ubiquitylation maximises the asymmetric localisation of core planar polarity proteins by fine-tuning their levels at junctions.
Planar cell polarity depends on the asymmetric localisation of core planar polarity proteins at apicolateral junctions. This asymmetric distribution probably develops through amplification of an initial asymmetry and seems to require the regulation of core protein levels. Now, Helen Strutt, Elizabeth Searle and co-workers (p. 1693) show that two distinct ubiquitylation pathways regulate the junctional levels and asymmetry of core planar polarity proteins in Drosophila. The researchers report that a Cullin-3-Diablo/Kelch ubiquitin ligase complex and the deubiquitylating enzyme Fat facets regulate the levels of the core planar polarity proteins Dishevelled and Flamingo, respectively, at apicolateral junctions but have no effect on the total cellular levels of Dishevelled and Flamingo. Notably, both increases and decreases in the junctional levels of core proteins caused by disruption of the ubiquitylation machinery reduce core protein asymmetry and disrupt planar cell polarity. Thus, the researchers suggest, ubiquitylation maximises the asymmetric localisation of core planar polarity proteins by fine-tuning their levels at junctions.
Tcf7l1 sets the stage for lineage specification
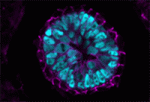 During mammalian embryogenesis, substantial cell proliferation occurs before the establishment of the body plan during gastrulation. Thus, before gastrulation, individual embryonic cells must be pluripotent. In vitro experiments with embryonic stem cells (ESCs) have indicated that the transcription factors Oct4, Sox2 and Nanog are components of a gene regulatory network (GRN) that stimulates self-renewal of pluripotent cells and have identified Tcf7l1 (Tcf3) as an inhibitor of GRN activity. But what is Tcf7l1’s function during embryonic development? To find out, Bradley Merrill and colleagues have been examining embryogenesis in Tcf7l1-/- mouse embryos (see p. 1665). They report that mesoderm specification is delayed in these embryos, thereby uncoupling it from primitive streak induction. Moreover, in vitro, Tcf7l1 activity is necessary to switch the response of pluripotent ESCs to Wnt/β-catenin signalling from self-renewal to mesoderm specification. Thus, the researchers suggest, Tcf7l1 prepares pluripotent epiblast cells in gastrulating mouse embryos for lineage specification and ensures that lineage specification is coordinated with the dynamic cellular events that occur during gastrulation.
During mammalian embryogenesis, substantial cell proliferation occurs before the establishment of the body plan during gastrulation. Thus, before gastrulation, individual embryonic cells must be pluripotent. In vitro experiments with embryonic stem cells (ESCs) have indicated that the transcription factors Oct4, Sox2 and Nanog are components of a gene regulatory network (GRN) that stimulates self-renewal of pluripotent cells and have identified Tcf7l1 (Tcf3) as an inhibitor of GRN activity. But what is Tcf7l1’s function during embryonic development? To find out, Bradley Merrill and colleagues have been examining embryogenesis in Tcf7l1-/- mouse embryos (see p. 1665). They report that mesoderm specification is delayed in these embryos, thereby uncoupling it from primitive streak induction. Moreover, in vitro, Tcf7l1 activity is necessary to switch the response of pluripotent ESCs to Wnt/β-catenin signalling from self-renewal to mesoderm specification. Thus, the researchers suggest, Tcf7l1 prepares pluripotent epiblast cells in gastrulating mouse embryos for lineage specification and ensures that lineage specification is coordinated with the dynamic cellular events that occur during gastrulation.
β-catenin helps ES cells stick to pluripotency
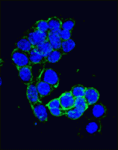 Canonical Wnt signalling and E-cadherin-mediated cell adhesion are both involved in mouse embryonic stem (mES) cell maintenance. β-catenin (Ctnnb1) is central to both these processes – it mediates the transactivation of Wnt target genes and also connects E-cadherin to the actin cytoskeleton via α-catenin. But which β-catenin function is absolutely required for mES cell self-renewal and pluripotency? On p. 1684, Ignacio del Valle and colleagues investigate this controversial question. The researchers use Ctnnb1-/-Eα mES cells in which the constitutive expression of an E-cadherin-α-catenin fusion protein maintains cell adhesion. Preservation of cell adhesion, the researchers report, is sufficient to promote the leukaemia inhibitory factor (Lif) signalling pathway, which is required for mES cell maintenance, and the transcriptional factor network that controls the mES cell state. They also implicate E-cadherin in the activation of Lif signalling by showing that it stabilises the Lifr-Gp130 co-receptor complex. Together, these findings suggest that only the adhesive function of β-catenin is absolutely required for the propagation of mES cells in culture.
Canonical Wnt signalling and E-cadherin-mediated cell adhesion are both involved in mouse embryonic stem (mES) cell maintenance. β-catenin (Ctnnb1) is central to both these processes – it mediates the transactivation of Wnt target genes and also connects E-cadherin to the actin cytoskeleton via α-catenin. But which β-catenin function is absolutely required for mES cell self-renewal and pluripotency? On p. 1684, Ignacio del Valle and colleagues investigate this controversial question. The researchers use Ctnnb1-/-Eα mES cells in which the constitutive expression of an E-cadherin-α-catenin fusion protein maintains cell adhesion. Preservation of cell adhesion, the researchers report, is sufficient to promote the leukaemia inhibitory factor (Lif) signalling pathway, which is required for mES cell maintenance, and the transcriptional factor network that controls the mES cell state. They also implicate E-cadherin in the activation of Lif signalling by showing that it stabilises the Lifr-Gp130 co-receptor complex. Together, these findings suggest that only the adhesive function of β-catenin is absolutely required for the propagation of mES cells in culture.
Hopx marks hair follicle stem cells
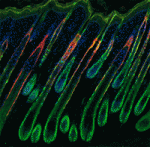 Adult tissue-specific stem cells both self-renew and generate functional progeny. Mammalian hair follicles, which are characterised by cyclical phases of growth (anagen), regression (catagen) and rest (telogen), are an ideal system in which to investigate the homeostasis of an adult stem cell population. On p. 1655, Jonathan Epstein and co-workers show that Hopx, which encodes an atypical homeodomain protein, is specifically expressed in long-lived stem cells in the basal bulge of the mouse telogen hair follicle. Hopx+ cells, they report, contribute to all the lineages of the mature hair follicle. In addition, the researchers identify a previously unknown progenitor population in the lower hair bulb of anagen-phase follicles and show that these Hopx-expressing cells contribute to the cytokeratin 6-positive inner bulge niche cells in telogen that regulate the quiescence of adjacent hair follicle stem cells. Because Hopx expression also marks other adult stem cell populations, the researchers suggest that tissue-specific stem cell populations might share homeostatic mechanisms.
Adult tissue-specific stem cells both self-renew and generate functional progeny. Mammalian hair follicles, which are characterised by cyclical phases of growth (anagen), regression (catagen) and rest (telogen), are an ideal system in which to investigate the homeostasis of an adult stem cell population. On p. 1655, Jonathan Epstein and co-workers show that Hopx, which encodes an atypical homeodomain protein, is specifically expressed in long-lived stem cells in the basal bulge of the mouse telogen hair follicle. Hopx+ cells, they report, contribute to all the lineages of the mature hair follicle. In addition, the researchers identify a previously unknown progenitor population in the lower hair bulb of anagen-phase follicles and show that these Hopx-expressing cells contribute to the cytokeratin 6-positive inner bulge niche cells in telogen that regulate the quiescence of adjacent hair follicle stem cells. Because Hopx expression also marks other adult stem cell populations, the researchers suggest that tissue-specific stem cell populations might share homeostatic mechanisms.
PLUS:
Brassinosteroid signalling
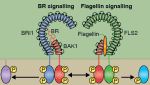 Zhi-Yong Wang and colleagues provide an overview of the highly integrated BR signalling network and explain how this steroid hormone functions as a master regulator of plant growth, development and metabolism.
Zhi-Yong Wang and colleagues provide an overview of the highly integrated BR signalling network and explain how this steroid hormone functions as a master regulator of plant growth, development and metabolism.
See the Development at a Glance poster article on p. 1615
Morphogen transport
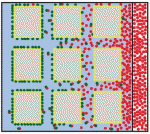 Alex Schier et al analyze various morphogen transport models using the morphogens Nodal, fibroblast growth factor and Decapentaplegic as case studies. They propose that most of the available data support the idea that morphogen gradients form by diffusion that is hindered by tortuosity and binding to extracellular molecules.
Alex Schier et al analyze various morphogen transport models using the morphogens Nodal, fibroblast growth factor and Decapentaplegic as case studies. They propose that most of the available data support the idea that morphogen gradients form by diffusion that is hindered by tortuosity and binding to extracellular molecules.
See the Hypothesis article on p. 1621


 (No Ratings Yet)
(No Ratings Yet)