On the origins of species-specific size
Posted by Jennifer Fish, on 25 February 2014
by Jennifer L. Fish and Richard A. Schneider
“For every type of animal there is a most convenient size, and a large change in size inevitably carries with it a change of form.”
Haldane 1926.
As articulated most eloquently by Haldane (1926) in his classic essay on “Being the Right Size”, every animal has it’s own particular size, which is ultimately linked to form, function, and fitness. Consequently, size is tightly regulated during development. To achieve proper structural integration, organs must keep track of size, as exemplified by the equal length of limbs. Right and left sides of the body develop independently, yet right and left limbs consistently reach comparable length (Allard & Tabin 2009). Mounting evidence suggests that developmental mechanisms regulating size are highly conserved and buffer variation (Leevers & McNeill 2005). So then how do size differences evolve?
In our recent manuscript, (Fish et al. 2014), we have addressed the question of organ size evolution from the perspective of the jaw. Utilizing two avian species, duck and quail, that exhibit remarkably different jaw size (Figure 1), we asked when, where, and how do duck acquire their long bills compared to quail who make short beaks? We began with the simple analogy that building a bigger structure such as a wall might involve using more bricks (rather than bigger bricks). Thus, we focused on the number of cellular precursors, which in this case is the cranial neural crest that gives rise to the jaw skeleton.
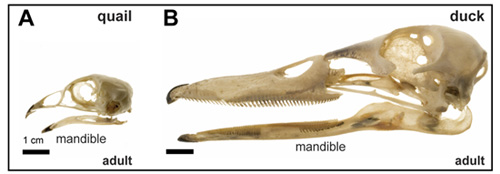
Figure 1: Species-specific differences adult quail (A) and duck (B) skulls showing species-specific differences in jaw size
We started by counting neural crest cells at several embryonic time points. We found that at a very early stage (HH8) the number of neural crest cells that are specified along the length of the neural folds is the same in quail and duck. But slightly later (HH10) when these cells begin to accumulate dorsally, duck have significantly more cells (15%) in the midbrain and rostral hindbrain region, which ultimately enables more duck cells to migrate into the presumptive jaw region by HH13. Remarkably, by HH20, duck have twice as many cells in their jaw primordia as do quail. To understand how an initial 15% difference could result in a doubling of the population by HH20, we analyzed cell proliferation and cell cycle length. We found that cell cycle length is longer in duck than quail, but when developmental rate is taken into account over absolute time, duck neural crest proliferate relatively faster than quail, which can explain the progressive increase in jaw size in duck embryos.
To uncover a mechanism through which duck increase the number of precursor cells that come out of the midbrain, we assayed for species-specific differences in the expression of brain regionalization markers. We compared Pax6 (forebrain), Otx2 (fore- and midbrain), Fgf8 (midbrain-hindbrain boundary), and Krox20 (r3 and r5 of the hindbrain) in duck and quail embryos at HH10. We found that duck and quail embryos have divergent brain shapes and spatial domains of gene expression. For example, duck embryos have a shorter and broader midbrain, which is also evidenced by a unique pattern of Otx2 expression (Figure 2A,B). Presumably, this broader duck midbrain congregates more neural crest cells in the region that will ultimately populate the jaw primordia. Interestingly, the Otx2 expression domain is already distinct in duck and quail embryos at HH6 (Figure 2C,D), suggesting that critical species-specific patterning mechanisms that affect jaw size may be in place from the earliest developmental stages.
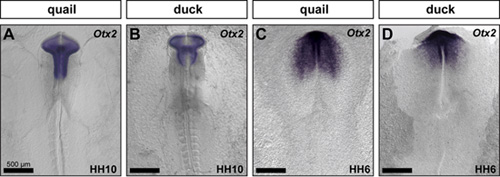
Figure 2: Species-specific differences appear early during development. Quail and duck embryos were compared molecularly by performing in situ hybridization for Otx2 at HH10 (A, B) and HH6 (C,D).
Overall, our work reveals that modifications to multiple aspects of cell biology, including the generation and allocation of neural crest cells destined to form the jaw skeleton, and species-specific regulation of cell proliferation, may underlie the evolution of jaw size.
References:
Allard, P. and Tabin, C. J. (2009). Achieving bilateral symmetry during vertebrate limb development. Semin. Cell Dev. Biol. 20, 479-484.
Fish JL, Sklar RS, Woronowicz KC, and Schneider RA. (2014). Multiple developmental mechanisms regulate species-specific jaw size. Development, 141:674-684.
Haldane, J. B. S. (1926). On Being the Right Size. Harper’s Magazine.
Leevers, S. J. and McNeill, H. (2005). Controlling the size of organs and organisms. Curr. Opin. Cell Biol. 17, 604-609.


 (6 votes)
(6 votes)