When F-actin dynamics and Hippo signalling activity meet to regulate tissue growth.
Posted by Florence Janody, on 11 May 2011
Genetic screens in flies brought me by chance to have a look at one of the basic apparatus of the cell: the actin cytoskeleton. At that time, I remembered my cell biology courses at University and since the actin cytoskeleton was not one of the hot spot, I though it was just a machinery required for basic cellular functions, from which, we knew almost everything. I quickly realized that in multicellular organisms, it was definitively worth to have a closer look at it.
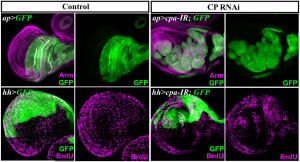
Given the crucial role for F-actin in numerous cellular processes, it came to us as a surprise that triggering excess F-actin polymerization, by disrupting the activity of the actin-Capping Protein (CP) heterodimer, did not automatically lead to cell lethality, but could trigger tissue growth. We therefore focused our efforts on investigating how the control of F-actin could be involved in preventing cell proliferation. This gave rise to our recent story published in Development ” Actin-Capping Protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila”.
We quickly realized that many of the targets genes controlled by the Hippo (Hpo) tumor suppressor pathway were upregulated in cells lacking CP. This conserved signal transduction pathway, has emerged as a critical regulator of tissue size both in Drosophila and mammals. Central to the Hpo pathway is a kinase cascade, which involves the Hpo and Warts (Wts) kinases and their adaptor proteins Salvador (Sav) and Mats. Phosphorylation of Wts by Hpo prevents nuclear translocation of the transcriptional co-activator Yorkie (Yki) through phosphorylation, leading to transcriptional downregulation of target genes that positively regulate cell growth, survival and proliferation. Multiple upstream inputs are known to regulate the core Hpo kinase cassette at various levels. However, how they do so is poorly understood (http://dev.biologists.org/content/138/1/9.abstract). We observed that in cells lacking CP, upregulation of Yki target genes was associated to a decrease in phosphorylated Yki and its relocalization to the nucleus. These behaviours resulted from defects in F-actin since knocking down the Cyclase associated protein Capulet (Capt), which sequesters actin monomers, also caused abnormal F-actin accumulation and ectopic Yki activity.
At around this time, we were happy to hear that the laboratory of Georg Halder had also identified CP and Capt in a S2 cells genome-wide RNAi screen for genes that inhibit expression of a Yki-dependent luciferase reporter gene. Their story, just published in EMBO Journal (Sansores-Garcia et al.), shows a sticking correlation between the F-actin levels and Hpo signalling output. While knocking down actin modulators that prevent F-actin accumulation triggers Yki activity, depleting actin regulators that promote F-actin accumulation has the opposite effect. Their work nicely shows that this link is conserved in mammalian cells. They also confirmed that extra F-actin polymerization of cells knocked down for CP or overexpressing an activated version of the actin nucleator Diaphanous (DiaCA), causes tissue growth in vivo through Yki activity and not through disruption of apical-basal cell polarity or signalling in general. Interestingly, dsRNAi targeting the Cofilin twinstar (tsr) was also tested positive as a modifier of Yki activity in their assays. However, in Drosophila epithelia, we found that loss of tsr had no effect on Yki target genes. Although, epithelial and S2 cells require both a proper F-actin network to regulate Hpo signalling activity, the different effects of tsr loss on Hpo signalling output suggest that Hpo signalling activity uses different F-actin networks in tissues and in single cells. In epithelia, CP, Capt and DiaCA control F-actin formation near the apical surface, while Tsr acts around the entire cell cortex. Because the integrity of the apical domain of epithelial cells seems critical for the maintenance of Hpo signalling, in epithelia, but not in S2 cells, a specialized population of polarized F-actin,regulated by CP, Capt and DiaCA at the apical cell membrane, may promote Hpo signalling activity.
One interesting issue was then to determine at what level of the pathway, CP or F-actin dynamics in general intersect with Hpo signalling activity. We observed that overexpressing Hpo or the upstream regulator Expanded (Ex) suppressed growth of CP-depleted cells, suggesting a role for F-actin upstream or in parallel to Ex. However, overexpressed, Ex and possibly Hpo, also suppressed F-actin accumulation of Cpa-depleted cells. Moreover, we found that Hpo signalling activity prevented F-actin accumulation, independently of Yki activity. We were therefore confronted to the problem: what is first, the egg or the chicken. Nevertheless, our results indicated an interdependency between Hpo signalling activity and F-actin dynamics in which CP and Hpo pathway activities inhibit F-actin accumulation, and the reduction in F-actin in turn sustains Hpo pathway activity, preventing Yki nuclear translocation and upregulation of proliferation and survival genes.
In contrast, Sansores et al. observed that the DiaCA-induced overgrowth was not suppressed by Hpo or Ex overexpression, whereas Wts could do so. Thus, they conclude that DiaCA and therefore F-actin affect the Hpo pathway upstream of Wts but in parallel to Ex and Hpo. Interestingly, they also show that, unlike loss of CP, the accumulation of F-actin caused by DiaCA overexpression was not suppressed by Wts, Ex, or Hpo overexpression. The different effects of DiaCA and CP loss on Hpo signalling activity, when Ex and Hpo are overexpressed, argue that the control of F-actin by Hpo pathway activity is required to sustain its activity. By preventing F-actin accumulation of CP-depleted cells, increased Ex or Hpo may sustain Hpo pathway activity. In contrast, because overexpressed Ex or Hpo cannot prevent excess F-actin resulting from DiaCA overexpression, F-actin accumulation can still inhibits Hpo pathway activity. Alternatively, the different outcome of cells depleted of CP or overexpressing DiaCA, when Ex or Hpo are overexpressed, could result from different strengths of the CP loss of function and DiaCA phenotypes on growth. The effect of expressing DiaCA on growth were stronger than those caused by loss of CP, suggesting that Hpo signalling activity is only partially affected by the loss of CP. Overexpressed Ex or Hpo might therefore counteract the mild effect of CP loss on Hpo signalling activity but not the one of DiaCA overexpression. Finally, it is possible that F-actin acts at several levels to regulate Hpo signalling activity. Consistent with this possibility, F-actin has also been shown to control the activity of the MST1/2 Hpo orthologs in mouse fibroblasts. Moreover, we noticed that clones of cells mutant for CP affected Hpo pathway activity cell autonomously but also non-autonomously. Thus, the control of F-actin by CP activity may have a dual function in controlling Hpo signalling activity. Sansores et al. did not observe any non-autonomous effect on Hpo signalling activity in cells knocked down for CP using RNAi, suggesting that the non-autonomous disruption of Hpo signalling activity is less sensitive to F-actin accumulation.
All these data took us to bring one more piece to the complex puzzle of how Hpo pathway activity is regulated and convinced us, more than ever, that different populations of F-actin filaments exist in the cell that have specialized functions. The challenge will now be to identify these populations, understand how they are regulated and characterize their role in controlling specific cellular events.
![]() Fernandez, B., Gaspar, P., Bras-Pereira, C., Jezowska, B., Rebelo, S., & Janody, F. (2011). Actin-Capping Protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila Development DOI: 10.1242/dev.063545
Fernandez, B., Gaspar, P., Bras-Pereira, C., Jezowska, B., Rebelo, S., & Janody, F. (2011). Actin-Capping Protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila Development DOI: 10.1242/dev.063545
Sansores-Garcia, L., Bossuyt, W., Wada, K., Yonemura, S., Tao, C., Sasaki, H., & Halder, G. (2011). Modulating F-actin organization induces organ growth by affecting the Hippo pathway The EMBO Journal DOI: 10.1038/emboj.2011.157


 (6 votes)
(6 votes)