In Development this week (Vol. 139, Issue 19)
Posted by Seema Grewal, on 4 September 2012
Here are the highlights from the current issue of Development:
Stem cells get cut loose
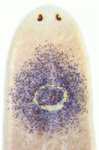 During tissue maintenance and regeneration, stem cells (SCs) are mobilised and migrate towards sites of tissue turnover or repair. However, owing to the inaccessible nature of most in vivo SC populations, very little is known about the molecular factors that regulate SC mobilisation. Otto Guedelhoefer and Alejandro Sánchez Alvarado tackle this problem by studying SC mobilisation during tissue homeostasis and regeneration in the planarian flatworm Schmidtea mediterranea (p. 3510). They show that planarian SCs migrate minimally in the absence of wounding; in partially irradiated animals, SCs do not migrate from the areas that were shielded from radiation to populate irradiated areas. Importantly, the researchers report, amputation increases SC dispersal and induces directional recruitment of SCs to sites of tissue repair, suggesting that factors capable of directing SCs are activated upon amputation. These findings highlight that, in planaria, SC depletion alone is not sufficient to trigger SC migration and that loss of tissue integrity is required to promote the directional migration of SCs.
During tissue maintenance and regeneration, stem cells (SCs) are mobilised and migrate towards sites of tissue turnover or repair. However, owing to the inaccessible nature of most in vivo SC populations, very little is known about the molecular factors that regulate SC mobilisation. Otto Guedelhoefer and Alejandro Sánchez Alvarado tackle this problem by studying SC mobilisation during tissue homeostasis and regeneration in the planarian flatworm Schmidtea mediterranea (p. 3510). They show that planarian SCs migrate minimally in the absence of wounding; in partially irradiated animals, SCs do not migrate from the areas that were shielded from radiation to populate irradiated areas. Importantly, the researchers report, amputation increases SC dispersal and induces directional recruitment of SCs to sites of tissue repair, suggesting that factors capable of directing SCs are activated upon amputation. These findings highlight that, in planaria, SC depletion alone is not sufficient to trigger SC migration and that loss of tissue integrity is required to promote the directional migration of SCs.
Coupling genome defence to epigenetic reprogramming
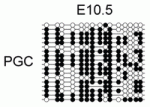 DNA methylation plays an important role in gene silencing and repressing transposable elements (TEs). During primordial germ cell (PGC) development, DNA methylation marks are erased during extensive epigenetic reprogramming, so how does this demethylation impact gene expression and TE repression in PGCs? Richard Meehan and co-workers (p. 3623) show that DNA methylation at the promoters of germline-specific genes couples genome-defence mechanisms to epigenetic reprogramming in mouse PGCs. The researchers identify a set of germline-specific genes that are dependent exclusively on promoter DNA methylation for their silencing; their promoters possess specialised chromatin in somatic cells that does not acquire additional repressive histone modifications. This set, they discover, is enriched in genes involved in suppressing TE activity in germ cells, and the expression of these genes is activated during two phases of DNA demethylation in PGCs. These findings suggest that unique reliance on promoter DNA methylation acts as a highly tuned sensor of global DNA demethylation and allows PGCs to be primed to suppress TEs.
DNA methylation plays an important role in gene silencing and repressing transposable elements (TEs). During primordial germ cell (PGC) development, DNA methylation marks are erased during extensive epigenetic reprogramming, so how does this demethylation impact gene expression and TE repression in PGCs? Richard Meehan and co-workers (p. 3623) show that DNA methylation at the promoters of germline-specific genes couples genome-defence mechanisms to epigenetic reprogramming in mouse PGCs. The researchers identify a set of germline-specific genes that are dependent exclusively on promoter DNA methylation for their silencing; their promoters possess specialised chromatin in somatic cells that does not acquire additional repressive histone modifications. This set, they discover, is enriched in genes involved in suppressing TE activity in germ cells, and the expression of these genes is activated during two phases of DNA demethylation in PGCs. These findings suggest that unique reliance on promoter DNA methylation acts as a highly tuned sensor of global DNA demethylation and allows PGCs to be primed to suppress TEs.
Stan points the way in planar polarity
 Many epithelial tissues display planar cell polarity (PCP). This phenomenon has been best studied in Drosophila in which most epidermal cells produce hairs at one side that all point in the same direction. The molecular mechanisms underlying PCP establishment remain controversial. Key players are the transmembrane proteins Starry night (Stan; also known as Flamingo), Frizzled (Fz) and Vang Gogh (Vang, also known as Strabismus). Stan, a protocadherin, forms homodimeric bridges between cells. These bridges appear to link Fz and Vang on the abutting distal and proximal faces of adjacent cells, and their resulting asymmetric distributions polarise both cells to point the same way. Now, Struhl, Casal and Lawrence (p. 3665) report the surprising finding that Vang is not essential for cell polarisation. Instead, asymmetric interactions between Stan and Stan/Fz are sufficient to define polarity, and Vang plays an accessory role, probably by enhancing the capacity of Stan to interact with Stan/Fz. These results challenge current models of PCP, although the authors propose an alternative that may reconcile the data.
Many epithelial tissues display planar cell polarity (PCP). This phenomenon has been best studied in Drosophila in which most epidermal cells produce hairs at one side that all point in the same direction. The molecular mechanisms underlying PCP establishment remain controversial. Key players are the transmembrane proteins Starry night (Stan; also known as Flamingo), Frizzled (Fz) and Vang Gogh (Vang, also known as Strabismus). Stan, a protocadherin, forms homodimeric bridges between cells. These bridges appear to link Fz and Vang on the abutting distal and proximal faces of adjacent cells, and their resulting asymmetric distributions polarise both cells to point the same way. Now, Struhl, Casal and Lawrence (p. 3665) report the surprising finding that Vang is not essential for cell polarisation. Instead, asymmetric interactions between Stan and Stan/Fz are sufficient to define polarity, and Vang plays an accessory role, probably by enhancing the capacity of Stan to interact with Stan/Fz. These results challenge current models of PCP, although the authors propose an alternative that may reconcile the data.
Predicting embryonic axes
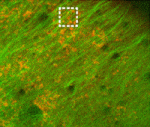 In many animals, the polarised transport of maternal factors by the microtubule cytoskeleton is required for setting up embryonic axes. Now, Karuna Sampath and colleagues show that microtubules at the vegetal cortex of early zebrafish embryos are dynamically remodelled and predict the future embryonic axis (p. 3644). Using live imaging, they find that two transient populations of microtubules – perpendicular bundles and parallel arrays – are detected exclusively at the vegetal cortex of fertilised embryos before the first cell division. The perpendicular bundles, which are likely to transport maternal factors via the yolk to the blastoderm, extend from the vegetal cortex and orient along the animal-vegetal axis. The parallel arrays form autonomously even in the absence of sperm entry and fertilisation. Importantly, the asymmetric orientation of parallel arrays shortly after fertilisation predicts where the zebrafish dorsal structures will form. Finally, the authors provide in vivo evidence for cortical rotation-like movements of cytoplasmic granules, similar to those occurring in Xenopus, suggesting that such movements might be more common in development than previously thought.
In many animals, the polarised transport of maternal factors by the microtubule cytoskeleton is required for setting up embryonic axes. Now, Karuna Sampath and colleagues show that microtubules at the vegetal cortex of early zebrafish embryos are dynamically remodelled and predict the future embryonic axis (p. 3644). Using live imaging, they find that two transient populations of microtubules – perpendicular bundles and parallel arrays – are detected exclusively at the vegetal cortex of fertilised embryos before the first cell division. The perpendicular bundles, which are likely to transport maternal factors via the yolk to the blastoderm, extend from the vegetal cortex and orient along the animal-vegetal axis. The parallel arrays form autonomously even in the absence of sperm entry and fertilisation. Importantly, the asymmetric orientation of parallel arrays shortly after fertilisation predicts where the zebrafish dorsal structures will form. Finally, the authors provide in vivo evidence for cortical rotation-like movements of cytoplasmic granules, similar to those occurring in Xenopus, suggesting that such movements might be more common in development than previously thought.
Potassium channels and patterning
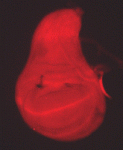 Mutations that disrupt the function of the inwardly rectifying Kir2.1 potassium channel cause developmental defects in both humans and mice, but the mechanisms underlying these abnormalities remain unclear. Now, on p. 3653, Emily Bates and co-workers show that disruption of a homologous Drosophila potassium channel, Irk2, causes developmental defects by modulating signalling of Decapentaplegic (Dpp), a bone morphogenetic protein (BMP) homologue. The authors find that compromised Irk2 function causes wing patterning defects similar to those observed when Dpp signalling is disrupted. The phenotypes of Irk2 mutant flies are enhanced by reducing Dpp function. Importantly, the researchers demonstrate that aberrant Irk2 function leads to a decrease in Dpp signalling within the wing: phosphorylation of Mad (a downstream effector of Dpp signalling) and the expression of spalt (a transcriptional target of Dpp) are decreased in Irk2 mutant wing discs. Collectively, these findings identify a novel mechanism by which potassium channels act during development and suggest that BMP pathways might somehow sense alterations in membrane potential.
Mutations that disrupt the function of the inwardly rectifying Kir2.1 potassium channel cause developmental defects in both humans and mice, but the mechanisms underlying these abnormalities remain unclear. Now, on p. 3653, Emily Bates and co-workers show that disruption of a homologous Drosophila potassium channel, Irk2, causes developmental defects by modulating signalling of Decapentaplegic (Dpp), a bone morphogenetic protein (BMP) homologue. The authors find that compromised Irk2 function causes wing patterning defects similar to those observed when Dpp signalling is disrupted. The phenotypes of Irk2 mutant flies are enhanced by reducing Dpp function. Importantly, the researchers demonstrate that aberrant Irk2 function leads to a decrease in Dpp signalling within the wing: phosphorylation of Mad (a downstream effector of Dpp signalling) and the expression of spalt (a transcriptional target of Dpp) are decreased in Irk2 mutant wing discs. Collectively, these findings identify a novel mechanism by which potassium channels act during development and suggest that BMP pathways might somehow sense alterations in membrane potential.
Insights into the origins of HSCs
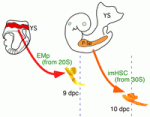 In mice, haematopoietic stem cells (HSCs) are first found in the dorsal aorta of early embryos shortly after 10.5 days post coitus (dpc) and in the foetal liver at 11 dpc. However, multipotent haematopoietic progenitors can also be detected in the dorsal aorta from 9 dpc. Do these cells contribute to adult haematopoiesis? Here, Ana Cumano and co-workers address this question by characterising this population of cells (p. 3521). The researchers show that multipotent progenitors detected in the dorsal aorta at 9 dpc, which they term immature HSCs (imHSCs), are endowed with long-term reconstitution capacity. Furthermore, they report, these cells are capable of evolving into HSCs under appropriate culture conditions and are able to colonise the mouse foetal liver. Of note, the early colonisation of the foetal liver by imHSCs precedes that of HSCs. Moreover, organ culture experiments suggest that, in the liver environment, imHSCs are able to mature into HSCs, thus identifying a novel stage in HSC development.
In mice, haematopoietic stem cells (HSCs) are first found in the dorsal aorta of early embryos shortly after 10.5 days post coitus (dpc) and in the foetal liver at 11 dpc. However, multipotent haematopoietic progenitors can also be detected in the dorsal aorta from 9 dpc. Do these cells contribute to adult haematopoiesis? Here, Ana Cumano and co-workers address this question by characterising this population of cells (p. 3521). The researchers show that multipotent progenitors detected in the dorsal aorta at 9 dpc, which they term immature HSCs (imHSCs), are endowed with long-term reconstitution capacity. Furthermore, they report, these cells are capable of evolving into HSCs under appropriate culture conditions and are able to colonise the mouse foetal liver. Of note, the early colonisation of the foetal liver by imHSCs precedes that of HSCs. Moreover, organ culture experiments suggest that, in the liver environment, imHSCs are able to mature into HSCs, thus identifying a novel stage in HSC development.
Plus…
An interview with Haruhiko Koseki
Primer: Epithelial-mesenchymal transitions: insights from development
Review: Tooth shape formation and tooth renewal: evolving with the same signals


 (No Ratings Yet)
(No Ratings Yet)