In Development this week (Vol. 141, Issue 7)
Posted by Seema Grewal, on 18 March 2014
Here are the highlights from the current issue of Development:
A vascular scaffold for islet nerve growth
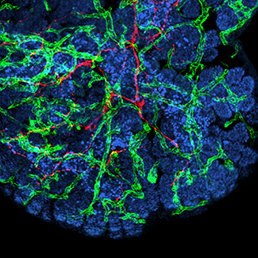 In many organs, the processes of vascularisation and innervation are frequently interdependent. In the pancreas, islet endocrine cells secrete vascular endocrine growth factor (VEGF) to direct vascularisation, but little is known about how islet innervation is regulated. On p. 1480, Marcela Brissova, Alvin Powers and colleagues analyse the role of VEGF, and the vasculature, in controlling nerve fibre growth in the developing mouse pancreas. They find that, although neural crest-derived nerves surround the islets during embryonic development, they do not penetrate them until postnatal stages. This late step of islet innervation is dependent on VEGF, but indirectly – via its effects on the vasculature. Axon guidance molecules and extracellular matrix factors expressed by endothelial cells, and growth factors produced by endocrine cells are likely involved in promoting innervation; the authors propose that the islet vessels may act as a scaffold for nerve fibre growth. Such insights into how islet formation, vascularisation and innervation are coordinated are key for developing strategies to produce functional islets for therapeutic purposes.
In many organs, the processes of vascularisation and innervation are frequently interdependent. In the pancreas, islet endocrine cells secrete vascular endocrine growth factor (VEGF) to direct vascularisation, but little is known about how islet innervation is regulated. On p. 1480, Marcela Brissova, Alvin Powers and colleagues analyse the role of VEGF, and the vasculature, in controlling nerve fibre growth in the developing mouse pancreas. They find that, although neural crest-derived nerves surround the islets during embryonic development, they do not penetrate them until postnatal stages. This late step of islet innervation is dependent on VEGF, but indirectly – via its effects on the vasculature. Axon guidance molecules and extracellular matrix factors expressed by endothelial cells, and growth factors produced by endocrine cells are likely involved in promoting innervation; the authors propose that the islet vessels may act as a scaffold for nerve fibre growth. Such insights into how islet formation, vascularisation and innervation are coordinated are key for developing strategies to produce functional islets for therapeutic purposes.
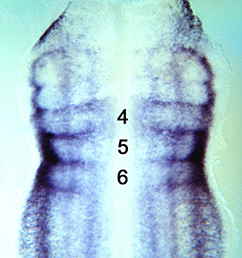
Sorting out the hindbrain with Hox
The Hox genes are best known as regulators of anterior-posterior identity along the embryonic axis, including in the central nervous system. Here (p. 1492), Alex Gould and co-workers identify a function for Hox proteins in regulating cell segregation and boundary formation between rhombomeres of the mammalian and chick hindbrain. Hox4 family members show an anterior expression border at the r6/7 boundary, and Hox4 depletion inhibits formation of this boundary. Conversely, cells ectopically expressing Hox4 cluster together and display boundary-like features at their borders. These data suggest that the presence of an interface between Hox4-expressing and non-expressing cells is required for cell segregation at the r6/r7 boundary. Mechanistically, the authors show that Eph/ephrin genes known to be involved in boundary formation are regulated by Hox4, and Hox4 appears to control cell shape in a non-autonomous fashion – promoting apical enlargement on either side of the rhombomere interface. Other Hox proteins appear to have similar activities, suggesting a conserved function for this protein family in regulating cell segregation.
Integrin internalisation in angiogenesis
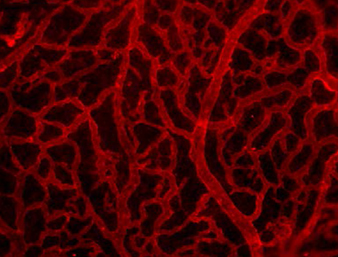 Clathrin-mediated endocytosis regulates the signalling activity and turnover of multiple plasma membrane proteins. Interfering with endocytosis can therefore have complex effects on developmental processes. William Sessa and colleagues (p. 1465) investigate the role of the Dynamin 2 (Dnm2) GTPase – a key component of the endocytic machinery – during angiogenesis in mice. Using endothelial cells in culture, they find that downregulation of Dnm2 impairs angiogenesis, even though vascular endothelial growth factor (VEGF) signalling is enhanced – due to increased surface levels of the receptor. This is in contrast to previous work suggesting that endocytosis might promote VEGF signalling. The authors ascribe the Dnm2 knockdown-induced defect in vessel formation at least partially to disrupted integrin turnover: focal adhesion size is increased and inactive integrin receptors appear to accumulate on the cell surface. Importantly, these observations hold true in vivo: conditional deletion of Dnm2 in mouse embryos causes severe angiogenic phenotypes that are consistent with impaired integrin function.
Clathrin-mediated endocytosis regulates the signalling activity and turnover of multiple plasma membrane proteins. Interfering with endocytosis can therefore have complex effects on developmental processes. William Sessa and colleagues (p. 1465) investigate the role of the Dynamin 2 (Dnm2) GTPase – a key component of the endocytic machinery – during angiogenesis in mice. Using endothelial cells in culture, they find that downregulation of Dnm2 impairs angiogenesis, even though vascular endothelial growth factor (VEGF) signalling is enhanced – due to increased surface levels of the receptor. This is in contrast to previous work suggesting that endocytosis might promote VEGF signalling. The authors ascribe the Dnm2 knockdown-induced defect in vessel formation at least partially to disrupted integrin turnover: focal adhesion size is increased and inactive integrin receptors appear to accumulate on the cell surface. Importantly, these observations hold true in vivo: conditional deletion of Dnm2 in mouse embryos causes severe angiogenic phenotypes that are consistent with impaired integrin function.
Measuring signal RAtio for limb patterning
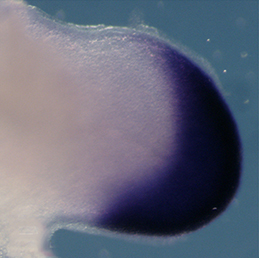 The vertebrate limb is an important model for understanding developmental patterning processes. Along the proximo-distal axis, the limb is segmented into stylopod (upper limb), zeugopod (lower limb) and autopod (hand/foot) regions, marked by the expression of particular homeobox genes – Meis1/2 most proximally and Hoxa13 most distally. Fibroblast growth factor (FGF) is a key inducer of distal fate, while the role of retinoic acid (RA) in promoting proximal fate has been controversial. Miguel Torres and co-workers (p. 1534) now show that RA can inhibit distal identity in both chick and mouse, and that downregulation of RA signalling by Meis1/2 is required to permit Hoxa13 expression and specify the autopod. Thus, opposing gradients of FGF and RA pattern the proximo-distal axis. However, distal cells only become competent to express Hoxa13 at later time points, and the authors provide evidence for a timing mechanism that involves regulation of the chromatin state. The authors therefore propose a dual mechanism for regulating proximo-distal identity: antagonistic signalling gradients and an underlying temporal constraint.
The vertebrate limb is an important model for understanding developmental patterning processes. Along the proximo-distal axis, the limb is segmented into stylopod (upper limb), zeugopod (lower limb) and autopod (hand/foot) regions, marked by the expression of particular homeobox genes – Meis1/2 most proximally and Hoxa13 most distally. Fibroblast growth factor (FGF) is a key inducer of distal fate, while the role of retinoic acid (RA) in promoting proximal fate has been controversial. Miguel Torres and co-workers (p. 1534) now show that RA can inhibit distal identity in both chick and mouse, and that downregulation of RA signalling by Meis1/2 is required to permit Hoxa13 expression and specify the autopod. Thus, opposing gradients of FGF and RA pattern the proximo-distal axis. However, distal cells only become competent to express Hoxa13 at later time points, and the authors provide evidence for a timing mechanism that involves regulation of the chromatin state. The authors therefore propose a dual mechanism for regulating proximo-distal identity: antagonistic signalling gradients and an underlying temporal constraint.
A new cell type in the frog skin
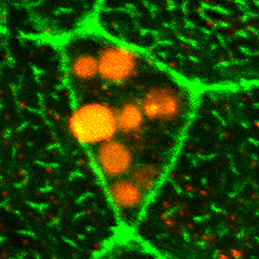 The Xenopus embryonic epidermis is a mucociliary epithelium – analogous to that found in mammalian airways. This tissue is characterised by the presence of multiciliated cells (MCCs) and goblet cells. A third cell type, the ion-secreting cell, was recently discovered in the Xenopus epidermis. Two papers now identify a final cell type in the frog embryonic skin, the small secretory cell (SSC). Eamon Dubaissi and colleagues (p. 1514) show that SSCs are specified by the transcription factor Foxa1, are characterised by the presence of large secretory vesicles containing mucin-like (glycosylated) proteins and are important for immune defence: tadpoles lacking SSCs die from bacterial infection. Axel Schweickert and co-workers (p. 1526) find that these cells also secrete serotonin and provide evidence for serotonin-mediated regulation of ciliary beat frequency in MCCs, as in other systems. Given the value of the Xenopus epidermis as a model for mucociliary epithelia and disease, the identification of SSCs provides important insights into the nature and function of this epithelium.
The Xenopus embryonic epidermis is a mucociliary epithelium – analogous to that found in mammalian airways. This tissue is characterised by the presence of multiciliated cells (MCCs) and goblet cells. A third cell type, the ion-secreting cell, was recently discovered in the Xenopus epidermis. Two papers now identify a final cell type in the frog embryonic skin, the small secretory cell (SSC). Eamon Dubaissi and colleagues (p. 1514) show that SSCs are specified by the transcription factor Foxa1, are characterised by the presence of large secretory vesicles containing mucin-like (glycosylated) proteins and are important for immune defence: tadpoles lacking SSCs die from bacterial infection. Axel Schweickert and co-workers (p. 1526) find that these cells also secrete serotonin and provide evidence for serotonin-mediated regulation of ciliary beat frequency in MCCs, as in other systems. Given the value of the Xenopus epidermis as a model for mucociliary epithelia and disease, the identification of SSCs provides important insights into the nature and function of this epithelium.
No master regulator for EMT
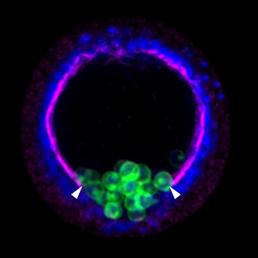 Epithelial-mesenchymal transition (EMT) is a cell state change used repeatedly during development across evolution, while aberrant EMT is associated with cancer metastasis. The transcriptional control of EMT has been extensively studied, and several transcription factors (TFs) shown to play crucial roles; notably, TFs such as Twist and Snail have been proposed to be ‘master regulators’ of EMT. On p. 1503, Lindsay Saunders and David McClay challenge this concept by analysing the specific functions of TFs implicated in the EMT gene regulatory network (GRN) of early sea urchin embryos. The authors analyse five features of EMT – basement membrane remodelling, de-adhesion, apical constriction, loss of apico-basal polarity and directed motility – and find that different TFs show varying effects on each of these processes. They then use these data to build sub-circuits within the GRN for each feature. Strikingly, none of the TFs are involved in all five sub-circuits, implying that – in sea urchin at least – the idea of a master regulator for EMT does not hold.
Epithelial-mesenchymal transition (EMT) is a cell state change used repeatedly during development across evolution, while aberrant EMT is associated with cancer metastasis. The transcriptional control of EMT has been extensively studied, and several transcription factors (TFs) shown to play crucial roles; notably, TFs such as Twist and Snail have been proposed to be ‘master regulators’ of EMT. On p. 1503, Lindsay Saunders and David McClay challenge this concept by analysing the specific functions of TFs implicated in the EMT gene regulatory network (GRN) of early sea urchin embryos. The authors analyse five features of EMT – basement membrane remodelling, de-adhesion, apical constriction, loss of apico-basal polarity and directed motility – and find that different TFs show varying effects on each of these processes. They then use these data to build sub-circuits within the GRN for each feature. Strikingly, none of the TFs are involved in all five sub-circuits, implying that – in sea urchin at least – the idea of a master regulator for EMT does not hold.
PLUS…
Haploid animal cells
 Haploid genetics holds great promise for understanding genome evolution and function. Much of the work on haploid genetics has previously been limited to microbes, but possibilities now extend to mammals. Here, Anton Wutz examines the potential use of haploid cells and puts them into a historical and biological context. See the Development at a Glance poster article on p. 1423
Haploid genetics holds great promise for understanding genome evolution and function. Much of the work on haploid genetics has previously been limited to microbes, but possibilities now extend to mammals. Here, Anton Wutz examines the potential use of haploid cells and puts them into a historical and biological context. See the Development at a Glance poster article on p. 1423
Switching on cilia: transcriptional networks regulating ciliogenesis
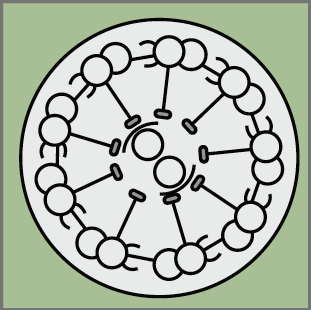 Cilia play many essential roles in fluid transport and cellular locomotion, and as sensory hubs for a variety of signal transduction pathways. Here, Sudipto Roy and colleagues review our understanding of the transcriptional control of ciliary biogenesis, highlighting the activities of FOXJ1 and the RFX family of transcriptional regulators. See the Review article on p. 1427
Cilia play many essential roles in fluid transport and cellular locomotion, and as sensory hubs for a variety of signal transduction pathways. Here, Sudipto Roy and colleagues review our understanding of the transcriptional control of ciliary biogenesis, highlighting the activities of FOXJ1 and the RFX family of transcriptional regulators. See the Review article on p. 1427


 (1 votes)
(1 votes)