In Development this week (Vol. 141, Issue 16)
Posted by Seema Grewal, on 6 August 2014
Here are the highlights from the current issue of Development:
PCP signalling is dispensable for neural crest migration
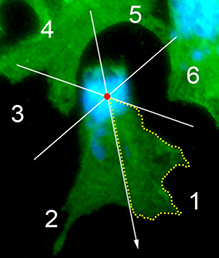 The neural crest (NC) is a transient and migratory population of cells that gives rise to a variety of cell types. During development, NC cells delaminate from the neural tube in a process that is closely coordinated with the process of neural tube closure, and studies have shown that signalling via the planar cell polarity (PCP) pathway is essential for both of these processes in Xenopus and zebrafish. However, it is unclear if PCP signalling is required for NC migration in mammals. Here, Andrew Copp and colleagues address this issue (p. 3153). They show that NC specification, migration and tissue colonisation are not perturbed in mice that lack the function of the core PCP protein Vangl2. Furthermore, they demonstrate that Vangl1 does not compensate for the loss of Vangl2, as Vangl1/Vangl2 double-mutant mice also exhibit normal NC migration. The NC-specific ablation of Vangl2 activity also has no effect on NC migration. Finally, the researchers demonstrate that the migratory properties of NC cells from wild-type and Vangl2 mutant neural tube explants cultured in vitro are indistinguishable. Together, these findings confirm that, in contrast to its essential role in neural tube closure, PCP signalling is not essential for NC migration. Importantly, these findings also suggest that PCP mutations are unlikely to be the cause of NC-related birth defects in humans.
The neural crest (NC) is a transient and migratory population of cells that gives rise to a variety of cell types. During development, NC cells delaminate from the neural tube in a process that is closely coordinated with the process of neural tube closure, and studies have shown that signalling via the planar cell polarity (PCP) pathway is essential for both of these processes in Xenopus and zebrafish. However, it is unclear if PCP signalling is required for NC migration in mammals. Here, Andrew Copp and colleagues address this issue (p. 3153). They show that NC specification, migration and tissue colonisation are not perturbed in mice that lack the function of the core PCP protein Vangl2. Furthermore, they demonstrate that Vangl1 does not compensate for the loss of Vangl2, as Vangl1/Vangl2 double-mutant mice also exhibit normal NC migration. The NC-specific ablation of Vangl2 activity also has no effect on NC migration. Finally, the researchers demonstrate that the migratory properties of NC cells from wild-type and Vangl2 mutant neural tube explants cultured in vitro are indistinguishable. Together, these findings confirm that, in contrast to its essential role in neural tube closure, PCP signalling is not essential for NC migration. Importantly, these findings also suggest that PCP mutations are unlikely to be the cause of NC-related birth defects in humans.
A helping Hand2 for heart development and regeneration
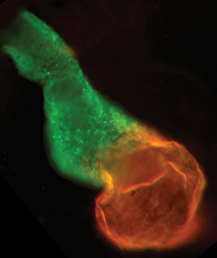 The production of cardiomyocytes is required both for embryonic heart formation and for cardiac regeneration following injury. The transcription factor Hand2 has been implicated in cardiomyocyte formation, but now, Deborah Yelon and colleagues demonstrate that Hand2 can in fact drive cardiomyocyte production in zebrafish (p.3112). They show that the overexpression of hand2 in early zebrafish embryos enhances the proliferation of cardiac progenitors within the second heart field, leading to increased numbers of cardiomyocytes and hence an increase in heart size. This cardiac enlargement also results from an increase in cardiomyocyte specification within the first heart field. Furthermore, they report, these effects require the phosphorylation-independent dimerization of Hand2 but not its direct binding to DNA. The researchers further investigate the role of Hand2 during regeneration and demonstrate that, in line with its role during development, hand2 overexpression can boost cardiomyocyte production following injury. These findings implicate the induction of hand2 expression as a key component of the cardiac regenerative response in zebrafish. Given that HAND2 has also been implicated in congenital heart disease (CHD) in humans, these findings also provide novel insights into the origins of CHD.
The production of cardiomyocytes is required both for embryonic heart formation and for cardiac regeneration following injury. The transcription factor Hand2 has been implicated in cardiomyocyte formation, but now, Deborah Yelon and colleagues demonstrate that Hand2 can in fact drive cardiomyocyte production in zebrafish (p.3112). They show that the overexpression of hand2 in early zebrafish embryos enhances the proliferation of cardiac progenitors within the second heart field, leading to increased numbers of cardiomyocytes and hence an increase in heart size. This cardiac enlargement also results from an increase in cardiomyocyte specification within the first heart field. Furthermore, they report, these effects require the phosphorylation-independent dimerization of Hand2 but not its direct binding to DNA. The researchers further investigate the role of Hand2 during regeneration and demonstrate that, in line with its role during development, hand2 overexpression can boost cardiomyocyte production following injury. These findings implicate the induction of hand2 expression as a key component of the cardiac regenerative response in zebrafish. Given that HAND2 has also been implicated in congenital heart disease (CHD) in humans, these findings also provide novel insights into the origins of CHD.
NR5A2: a central player in pancreas development
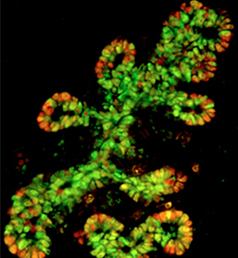 NR5A2 is an orphan nuclear hormone receptor that has diverse developmental and physiological functions. It is expressed in the inner cell mass of early mouse embryos and in the developing endoderm, but its role in organogenesis is unclear. Here, Ray MacDonald and co-workers reveal a crucial role for NR5A2 during pancreatic development in mice (p.3123). They first show that Nr5a2 is highly expressed in multipotent progenitor cells (MPCs), which give rise to endocrine, acinar and ductal cells, and in pre-MPCs, consistent with a role for NR5A2 in MPC formation. Indeed, pancreas-specific inactivation of Nr5a2 greatly diminishes the number of MPCs, and the development of all three lineages is affected. Subsequently, the researchers report, Nr5a2 expression is maintained in pre-acinar, acinar and ductal cells but is reduced in islet cells, suggesting that it regulates the development of the acinar lineage. In line with this, acinar morphogenesis was shown to be defective in an NR5A2-deficient pancreas. Furthermore, gene expression analyses indicate that NR5A2 can directly and indirectly modulate the expression of many genes involved in acinar differentiation and cell cycle control as well as in branching morphogenesis. These results demonstrate that NR5A2 controls multiple aspects of pancreas development and suggest that the experimental modulation of NR5A2 activity could be used to strategically direct the formation of pancreatic -cells in vitro.
NR5A2 is an orphan nuclear hormone receptor that has diverse developmental and physiological functions. It is expressed in the inner cell mass of early mouse embryos and in the developing endoderm, but its role in organogenesis is unclear. Here, Ray MacDonald and co-workers reveal a crucial role for NR5A2 during pancreatic development in mice (p.3123). They first show that Nr5a2 is highly expressed in multipotent progenitor cells (MPCs), which give rise to endocrine, acinar and ductal cells, and in pre-MPCs, consistent with a role for NR5A2 in MPC formation. Indeed, pancreas-specific inactivation of Nr5a2 greatly diminishes the number of MPCs, and the development of all three lineages is affected. Subsequently, the researchers report, Nr5a2 expression is maintained in pre-acinar, acinar and ductal cells but is reduced in islet cells, suggesting that it regulates the development of the acinar lineage. In line with this, acinar morphogenesis was shown to be defective in an NR5A2-deficient pancreas. Furthermore, gene expression analyses indicate that NR5A2 can directly and indirectly modulate the expression of many genes involved in acinar differentiation and cell cycle control as well as in branching morphogenesis. These results demonstrate that NR5A2 controls multiple aspects of pancreas development and suggest that the experimental modulation of NR5A2 activity could be used to strategically direct the formation of pancreatic -cells in vitro.
Auxin keeps stomata in the dark
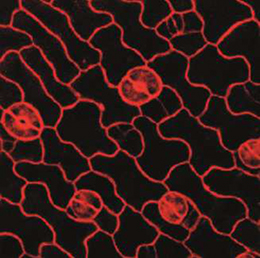 The development of stomata – the epidermal pores on plant leaves that regulate gas exchange – is tightly regulated by various environmental factors. Light, for example, promotes stomatal development; very few stomata are found on the epidermis of dark-grown seedlings. Here, on p.3165, Ute Hoecker and colleagues report that auxin, acting via Aux/IAA proteins, plays a key role in repressing stomatal development in dark-grown seedlings. The researchers show that aux/iaa mutants, which display auxin insensitivity, exhibit excessive stomata production specifically in dark-grown seedlings. This stomata-overproducing phenotype is also observed in mutants that are defective in auxin biosynthesis or perception, suggesting that auxin acts to repress stomatal production in the dark. They could further show that the excessive formation of stomata is caused by an increase in cell divisions within the stomatal lineage. Finally, the researchers use a combination of epistasis studies to elucidate a genetic network that integrates light and auxin signals in order to regulate stomatal development.
The development of stomata – the epidermal pores on plant leaves that regulate gas exchange – is tightly regulated by various environmental factors. Light, for example, promotes stomatal development; very few stomata are found on the epidermis of dark-grown seedlings. Here, on p.3165, Ute Hoecker and colleagues report that auxin, acting via Aux/IAA proteins, plays a key role in repressing stomatal development in dark-grown seedlings. The researchers show that aux/iaa mutants, which display auxin insensitivity, exhibit excessive stomata production specifically in dark-grown seedlings. This stomata-overproducing phenotype is also observed in mutants that are defective in auxin biosynthesis or perception, suggesting that auxin acts to repress stomatal production in the dark. They could further show that the excessive formation of stomata is caused by an increase in cell divisions within the stomatal lineage. Finally, the researchers use a combination of epistasis studies to elucidate a genetic network that integrates light and auxin signals in order to regulate stomatal development.
PLUS…
Out with the old, in with the new
Morpholino oligomers have been widely used by the zebrafish community for many years to generate loss-of-function phenotypes. Stefan Schulte-Merker and Didier Stainier reassess the usefulness of this methodology in light of recent developments in genome editing technologies. See the Spotlight on p.3103
Circadian clock-mediated control of stem cells
 Recent research suggests that circadian clock mechanisms control more than just daily timekeeping. Here, Steven Brown discusses how such mechanisms can influence stem cell biology and hence tissue development, homeostasis and regeneration. See the Review on p.3105
Recent research suggests that circadian clock mechanisms control more than just daily timekeeping. Here, Steven Brown discusses how such mechanisms can influence stem cell biology and hence tissue development, homeostasis and regeneration. See the Review on p.3105


 (No Ratings Yet)
(No Ratings Yet)