In Development this week (Vol. 142, Issue 9)
Posted by Seema Grewal, on 28 April 2015
Here are the highlights from the current issue of Development:
The light at the end of the tubule
 Tubular structures, such as kidney tubules or blood vessels, carry out crucial functions in organisms. Their morphogenesis requires an orchestrated sequence of cellular rearrangements, the disruption of which leads to tubule dysfunction, as observed in polycystic kidney disease. While the initiation of lumen formation is well understood, less is known about the process of lumen expansion. Using time-lapse analysis of the Ciona intestinalis notochord, a simple model of tubulogenesis in which a lumen forms between two cells connected by an apical ring of cell-cell junctions, Di Jiang and co-workers (p. 1639) show that lumen growth is a non-linear process: it exhibits a lag phase, where the lumen continues to grow but the tight junction ring does not expand. These peculiar kinetics are regulated by the contractility and the dynamics of actomyosin filaments at the lateral cell-cell junctions. Mechanistically, TGFβ negatively regulates the enlargement of the tight junction ring through inhibition of the RhoA-ROCK pathway, which reduces actomyosin contractility. After the lag phase, the osmotic pressure created in the lumen by the apically localised anion transporter Slc26 allows the enlargement of the junctional ring to resume. This study reveals unexpected and complex lumen expansion kinetics, furthering our knowledge of tubule morphogenesis.
Tubular structures, such as kidney tubules or blood vessels, carry out crucial functions in organisms. Their morphogenesis requires an orchestrated sequence of cellular rearrangements, the disruption of which leads to tubule dysfunction, as observed in polycystic kidney disease. While the initiation of lumen formation is well understood, less is known about the process of lumen expansion. Using time-lapse analysis of the Ciona intestinalis notochord, a simple model of tubulogenesis in which a lumen forms between two cells connected by an apical ring of cell-cell junctions, Di Jiang and co-workers (p. 1639) show that lumen growth is a non-linear process: it exhibits a lag phase, where the lumen continues to grow but the tight junction ring does not expand. These peculiar kinetics are regulated by the contractility and the dynamics of actomyosin filaments at the lateral cell-cell junctions. Mechanistically, TGFβ negatively regulates the enlargement of the tight junction ring through inhibition of the RhoA-ROCK pathway, which reduces actomyosin contractility. After the lag phase, the osmotic pressure created in the lumen by the apically localised anion transporter Slc26 allows the enlargement of the junctional ring to resume. This study reveals unexpected and complex lumen expansion kinetics, furthering our knowledge of tubule morphogenesis.
Tuning into cilium-mediated Shh transduction
 The primary cilium is an antenna-like structure present at the surface of most cells and necessary for normal development. In particular, it is required for Shh signalling, a crucial developmental pathway. However, the molecular mechanisms underlying the connection between the cilium and Shh transduction remain elusive. To address whether the ciliary localisation of Gli2, a transcriptional effector of the Shh pathway, is required for its activation, Aimin Liu and colleagues (p.1651) generated a knock-in mouse strain in which Gli2ΔCLR, a non-ciliary variant of Gli2 that retains transcriptional activity and responds to its inhibitor Sufuin vitro, is expressed in a similar pattern to endogenous Gli2. Shh signalling is compromised in Gli2ΔCLR mutants and is not restored by the pharmacological activation of Smo, the co-receptor that transduces the Shh signal, in vitro or by the depletion of Ptc1, the Shh receptor that inhibits Smo, in vivo. Furthermore, the authors show that Gli2ΔCLR activates Shh targets at the same level as endogenous Gli2 in the absence of Sufu, indicating that the impaired Shh signalling observed in Gli2ΔCLR mutants results from an impaired ciliary-dependent release from Sufu inhibition. In summary, these findings reveal that the ciliary localisation of Gli2 is required for its activation, highlighting the importance of the primary cilium as a signalling compartment.
The primary cilium is an antenna-like structure present at the surface of most cells and necessary for normal development. In particular, it is required for Shh signalling, a crucial developmental pathway. However, the molecular mechanisms underlying the connection between the cilium and Shh transduction remain elusive. To address whether the ciliary localisation of Gli2, a transcriptional effector of the Shh pathway, is required for its activation, Aimin Liu and colleagues (p.1651) generated a knock-in mouse strain in which Gli2ΔCLR, a non-ciliary variant of Gli2 that retains transcriptional activity and responds to its inhibitor Sufuin vitro, is expressed in a similar pattern to endogenous Gli2. Shh signalling is compromised in Gli2ΔCLR mutants and is not restored by the pharmacological activation of Smo, the co-receptor that transduces the Shh signal, in vitro or by the depletion of Ptc1, the Shh receptor that inhibits Smo, in vivo. Furthermore, the authors show that Gli2ΔCLR activates Shh targets at the same level as endogenous Gli2 in the absence of Sufu, indicating that the impaired Shh signalling observed in Gli2ΔCLR mutants results from an impaired ciliary-dependent release from Sufu inhibition. In summary, these findings reveal that the ciliary localisation of Gli2 is required for its activation, highlighting the importance of the primary cilium as a signalling compartment.
Shaping the cerebellum
 The cerebellum, a posterior part of the brain crucial for motor coordination, is composed of folia – functionally distinct units that each receive specific combinations of inputs from the rest of the nervous system. Generation of folia, separated by fissures with anchoring centres at their base, requires the proliferation of granule cell progenitors (gcps) and their differentiation into granule cells (gcs). The basic pattern of folia (relative size, number) is conserved across species. To investigate how gcp behaviour regulates folium geometry, Alexandra Joyner and co-workers (p. 1661) performed a clonal analysis and evaluated the geometry of gc clones, their cell number and cell dispersion with respect to folium size and timing of generation. Gcp division and dispersion were preferentially oriented along the anterior-posterior (AP) axis, which correlates with the preferential AP growth of the cerebellum. Furthermore, gcps do not cross anchoring centres and show different behaviour (geometry and cell number) if they reside in short folia versus long folia. Lastly, by analysing clone geometry in En1+/−;En2−/− mice with shorter folia, the authors show that gcp behaviour does underlie folia size. This study uncovers how the behaviour of neuronal progenitors shapes cerebellar foliation, providing crucial insight into the possible mechanisms that lead to the emergence of a folded neocortex in gyrencephalic mammals.
The cerebellum, a posterior part of the brain crucial for motor coordination, is composed of folia – functionally distinct units that each receive specific combinations of inputs from the rest of the nervous system. Generation of folia, separated by fissures with anchoring centres at their base, requires the proliferation of granule cell progenitors (gcps) and their differentiation into granule cells (gcs). The basic pattern of folia (relative size, number) is conserved across species. To investigate how gcp behaviour regulates folium geometry, Alexandra Joyner and co-workers (p. 1661) performed a clonal analysis and evaluated the geometry of gc clones, their cell number and cell dispersion with respect to folium size and timing of generation. Gcp division and dispersion were preferentially oriented along the anterior-posterior (AP) axis, which correlates with the preferential AP growth of the cerebellum. Furthermore, gcps do not cross anchoring centres and show different behaviour (geometry and cell number) if they reside in short folia versus long folia. Lastly, by analysing clone geometry in En1+/−;En2−/− mice with shorter folia, the authors show that gcp behaviour does underlie folia size. This study uncovers how the behaviour of neuronal progenitors shapes cerebellar foliation, providing crucial insight into the possible mechanisms that lead to the emergence of a folded neocortex in gyrencephalic mammals.
Ignoring RA to maintain the germline stem cell pool
 In tissues, niche-derived signals promote stem cell self-renewal and the spatially restricted environment shields stem cells from differentiating signals, thus maintaining the stem cell pool. In an open niche environment, such as the seminiferous tubules, where both self-renewal and differentiating signals are ubiquitously distributed, it is unclear how stem cells are maintained in an undifferentiated state. In this study, Shosei Yoshida and colleagues (p. 1582) use lineage-tracing analysis to show that retinoic acid (RA) induces the differentiation of a subpopulation of germ cells marked by NGN3, while another subpopulation (GFRα1+) is maintained. This differential response to RA is explained by the fact that the expression of RARγ, the RA receptor, is restricted to the NGN3+ cell population, thus conferring its differentiation capacity. Interestingly, forced expression of RARγ in GFRα1+cells provided them with the competence to differentiate. This study reveals that the selective response of different germ stem cell populations to RA preserves an undifferentiated stem cell pool, allowing spermatogenesis to persist throughout life.
In tissues, niche-derived signals promote stem cell self-renewal and the spatially restricted environment shields stem cells from differentiating signals, thus maintaining the stem cell pool. In an open niche environment, such as the seminiferous tubules, where both self-renewal and differentiating signals are ubiquitously distributed, it is unclear how stem cells are maintained in an undifferentiated state. In this study, Shosei Yoshida and colleagues (p. 1582) use lineage-tracing analysis to show that retinoic acid (RA) induces the differentiation of a subpopulation of germ cells marked by NGN3, while another subpopulation (GFRα1+) is maintained. This differential response to RA is explained by the fact that the expression of RARγ, the RA receptor, is restricted to the NGN3+ cell population, thus conferring its differentiation capacity. Interestingly, forced expression of RARγ in GFRα1+cells provided them with the competence to differentiate. This study reveals that the selective response of different germ stem cell populations to RA preserves an undifferentiated stem cell pool, allowing spermatogenesis to persist throughout life.
PLUS:
Animal models for studying neural crest development: is the mouse different?
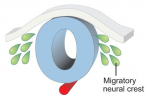 The neural crest is a uniquely vertebrate cell type and has been well studied in a number of model systems. Here, Bariga, Trainor, Bronner and Mayor discuss species-specific differences in neural crest development, urging the community to consider these differences and highlighting the need for further research in complementary systems. See the Spotlight on p. 1555
The neural crest is a uniquely vertebrate cell type and has been well studied in a number of model systems. Here, Bariga, Trainor, Bronner and Mayor discuss species-specific differences in neural crest development, urging the community to consider these differences and highlighting the need for further research in complementary systems. See the Spotlight on p. 1555
Sensory hair cell development and regeneration: similarities and differences
 Sensory hair cells are mechanoreceptors of the auditory and vestibular systems and are crucial for hearing and balance. Auditory hair cells in adult mammals are unable to regenerate whereas hair cells in the chick cochlea and the zebrafish lateral line are, prompting studies into the factors that regulate hair cell development and regeneration in various species. Here, Cheng and co-workers review these studies. See the Review on p. 1561
Sensory hair cells are mechanoreceptors of the auditory and vestibular systems and are crucial for hearing and balance. Auditory hair cells in adult mammals are unable to regenerate whereas hair cells in the chick cochlea and the zebrafish lateral line are, prompting studies into the factors that regulate hair cell development and regeneration in various species. Here, Cheng and co-workers review these studies. See the Review on p. 1561
Intrinsic and extrinsic mechanisms regulating satellite cell function
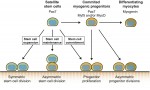 Muscle stem cells, termed satellite cells, are crucial for skeletal muscle growth and regeneration. Here, Rudnicki and colleagues review recent discoveries of the intrinsic and extrinsic factors that regulate satellite cell behaviour in regenerating and degenerating muscles. See the Review on p. 1572
Muscle stem cells, termed satellite cells, are crucial for skeletal muscle growth and regeneration. Here, Rudnicki and colleagues review recent discoveries of the intrinsic and extrinsic factors that regulate satellite cell behaviour in regenerating and degenerating muscles. See the Review on p. 1572


 (No Ratings Yet)
(No Ratings Yet)