Cell motion associated with stemness
Posted by Daisuke Nanba, on 14 May 2015
Stem cells play crucial roles in development as well as tissue homeostasis, repair, and regeneration, and their dysregulation is involved in diseases and aging of the tissues. The stem cell is defined as a cell that has the ability to self-renew and also to produce differentiated progeny for a long-term. Yet, stem cells require other functional properties to regulate a variety of biological processes.
Epidermal keratinocyte stem cells are one of well-characterized somatic stem cells in human and successfully applied for autologous transplantation of epidermal sheets onto patients with extensive burns and genetic disorders. However, keratinocyte stem cells are eventually converted into keratinocytes with more restricted proliferative capacity. So we lose the stem cells during serial cultivation, and as a result, sometimes fail the transplantation. Therefore, a noninvasive quality control of the stem cell culture is essential for successful regenerative medicine.
We have previously found that cultured human keratinocyte stem cells and transient amplifying (TA) cells differ in their actin filament organization. This causes the two distinct modes of epidermal growth factor (EGF)-induced colony dynamics in colonies formed by keratinocyte stem cells and TA cells1. So, we decided to explore the relationship between spontaneous cell motion and proliferative capacity in cultured human keratinocytes to identify the stem cells noninvasively and in situ.
As described in our resent paper2, we found that keratinocytes with significant proliferative capacity displayed a unique rotational motion at the two-cell stage colony which is formed by a single cell division of individual keratinocytes (Figure 1). We then simulated the interaction of a hundred rotating keratinocytes with physics of multiparticle systems and proved that this rotational motion of cells gives rise to collective motion of keratinocytes in the colony (Figure 2). This in silico experiments also revealed the possibility of predicting a keratinocyte stem cell colony by only measuring cell locomotion speed. We then validated this prediction with a combination of time-lapse observation and clonal analysis with human keratinocyte stem cell culture system, and finally, we could identify human keratinocyte stem cell colonies with a noninvasive procedure.
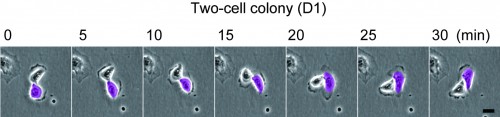
Figure 1
Rotational motion of a two-cell colony oh human keratinocytes.
Human epidermal keratinocytes were seeded on one fourth of normal number of feeder cells. The two-cell colonies of keratinocytes (paired keratinocytes) were observed 1 day later. Bar, 20 um.
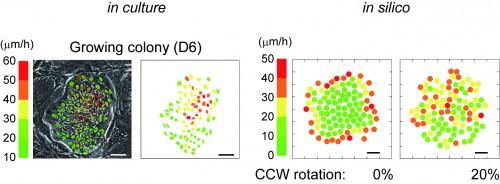
Figure 2
In silico reconstruction of keratinocyte stem cell colony dynamics.
Left panel shows that distribution of locomotion speed of keratinocytes in a growing colony after 6 days cultivation. Each color indicates different locomotion speeds that were calculated with 3 hours time-lapse observation. Bar 100 um. Right panel shows that distribution of locomotion speed of keratinocytes in a growing colony in silico. The speed of each cells was calculated by a simulation experiment based on our modeling. Bar, 50 um.
We also found that alpha6 integrin, which is a known marker of keratinocyte stem cells, is required for rotational and collective motion of keratinocytes. alpha6 integrin is also highly expressed at the leading edge of migrating epidermis during wound repair. Although the mechanism of the rotational motion associated with growth capacity remains unclear, motile epithelial cells could retain the significant proliferative capacity, or a population of migrating cells might contain stem cells. It seems to be involved in development as well as tissue repair and regeneration.
References
1. Nanba, D., Toki, F., Matsushita, N., Matsushita, S., Higashiyama, S., & Barrandon, Y. (2013). Actin filament dynamics impacts keratinocyte stem cell maintenance EMBO Molecular Medicine, 5 (4), 640-653 DOI: 10.1002/emmm.201201839
2. Nanba, D., Toki, F., Tate, S., Imai, M., Matsushita, N., Shiraishi, K., Sayama, K., Toki, H., Higashiyama, S., & Barrandon, Y. (2015). Cell motion predicts human epidermal stemness The Journal of Cell Biology, 209 (2), 305-315 DOI: 10.1083/jcb.201409024


 (4 votes)
(4 votes)