In Development this week (Vol. 142, Issue 12)
Posted by Seema Grewal, on 16 June 2015
Here are the highlights from the current issue of Development:
New tool for myelin formation in vitro
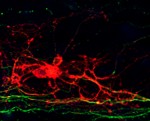 The myelination of axons by oligodendrocytes in the nervous system is crucial for neuron function and survival. Its disruption leads to permanent functional defects, as seen in numerous severe neurological pathologies. In order to study the developmental principles of this process and develop effective regenerative strategies, Fred Gage and colleagues have developed a system that allows robust and consistent myelination in vitro (see p. 2213). Working with mouse embryonic stem cell cultures, the authors used established protocols to generate cortical neurons, and developed a new method to produce myelinating oligodendrocytes. By co-culturing such cells in a microfluidic device that allows long-term imaging and analysing them using computer-assisted evaluation of myelin formation (which minimises human intervention), they showed that oligodendrocyte processes anchor to axons before wrapping them. Combining their results with previously published data, the authors establish a new model of myelination whereby oligodendrocytes anchor to bare axons, wrap around them and then form a myelin sheet. This study provides insight into the temporal sequence of the myelination process and offers a valuable tool to develop future regenerative strategies.
The myelination of axons by oligodendrocytes in the nervous system is crucial for neuron function and survival. Its disruption leads to permanent functional defects, as seen in numerous severe neurological pathologies. In order to study the developmental principles of this process and develop effective regenerative strategies, Fred Gage and colleagues have developed a system that allows robust and consistent myelination in vitro (see p. 2213). Working with mouse embryonic stem cell cultures, the authors used established protocols to generate cortical neurons, and developed a new method to produce myelinating oligodendrocytes. By co-culturing such cells in a microfluidic device that allows long-term imaging and analysing them using computer-assisted evaluation of myelin formation (which minimises human intervention), they showed that oligodendrocyte processes anchor to axons before wrapping them. Combining their results with previously published data, the authors establish a new model of myelination whereby oligodendrocytes anchor to bare axons, wrap around them and then form a myelin sheet. This study provides insight into the temporal sequence of the myelination process and offers a valuable tool to develop future regenerative strategies.
Fishing for clues on wound repair
 After an acute wound, tight regulation of repair signalling pathways is essential to ensure wound resolution and avoid chronic tissue damage. Interestingly, the molecular signals induced during wound healing are also present in chronic wounds but their specific roles in each situation remain mysterious. In order to identify factors that contribute to chronic tissue damage, Anna Huttenlocher and co-workers (p.2136) studied two zebrafish models of chronic epithelial damage and inflammation, the hai1 and clint1 mutants. These mutants exhibited disorganised collagen fibres, the deposition of which is a key step during normal repair, upregulated expression of mmp9, a matrix metalloproteinase that degrades collagen fibres during the remodelling phase, and a defective recruitment of leukocytes – cells that clear pathogens and debris. mmp9 depletion partially restored collagen organisation in hai1defective animals, but in control fish it impaired acute wound healing, specifically the change in collagen structure normally seen during repair and leukocyte recruitment. Mechanistically, mmp9 expression is induced during acute injury by NFκB, a known regulator of mmp9 expression in other systems. This study highlights the importance of tightly controlling Mmp9 activity, since it differentially regulates acute and chronic tissue damage and repair.
After an acute wound, tight regulation of repair signalling pathways is essential to ensure wound resolution and avoid chronic tissue damage. Interestingly, the molecular signals induced during wound healing are also present in chronic wounds but their specific roles in each situation remain mysterious. In order to identify factors that contribute to chronic tissue damage, Anna Huttenlocher and co-workers (p.2136) studied two zebrafish models of chronic epithelial damage and inflammation, the hai1 and clint1 mutants. These mutants exhibited disorganised collagen fibres, the deposition of which is a key step during normal repair, upregulated expression of mmp9, a matrix metalloproteinase that degrades collagen fibres during the remodelling phase, and a defective recruitment of leukocytes – cells that clear pathogens and debris. mmp9 depletion partially restored collagen organisation in hai1defective animals, but in control fish it impaired acute wound healing, specifically the change in collagen structure normally seen during repair and leukocyte recruitment. Mechanistically, mmp9 expression is induced during acute injury by NFκB, a known regulator of mmp9 expression in other systems. This study highlights the importance of tightly controlling Mmp9 activity, since it differentially regulates acute and chronic tissue damage and repair.
Closing in on insect cellularisation
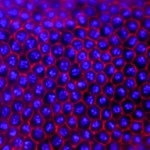 In most insects, the initial phase of embryogenesis involves multiple nuclear divisions to generate a syncytium, migration of the resulting nuclei to the cell cortex, followed by cellularisation. This last process has been thoroughly studied in Drosophila: the plasma membrane invaginates around the nuclei and extends to generate a basal membrane, forming a layer of epithelial cells. To what extent is this process conserved in other insects? To investigate this (see p.2173), Maurijn van der Zee and colleagues studied cellularisation in Tribolium castaneum, a beetle that has more ancestral traits than Drosophila. Among other differences, the authors found previously undescribed junctions linking the extending basal membrane to the forming yolk membrane. To identify the nature of these junctions, they performed a parental RNAi screen and found that the disruption of Innexin7a (Inx7a), whose Drosophila orthologue is dispensable for cellularisation, significantly impairs basal cell closure during Tribolium castaneum cellularisation. Inx7a is localised at the basal membrane of the forming epithelium and is required for its formation and for the stabilisation of the invaginated membrane. This study provides insight into the mechanisms of cellularisation in a non-Drosophila insect model, which are likely to be conserved in a greater number of insects.
In most insects, the initial phase of embryogenesis involves multiple nuclear divisions to generate a syncytium, migration of the resulting nuclei to the cell cortex, followed by cellularisation. This last process has been thoroughly studied in Drosophila: the plasma membrane invaginates around the nuclei and extends to generate a basal membrane, forming a layer of epithelial cells. To what extent is this process conserved in other insects? To investigate this (see p.2173), Maurijn van der Zee and colleagues studied cellularisation in Tribolium castaneum, a beetle that has more ancestral traits than Drosophila. Among other differences, the authors found previously undescribed junctions linking the extending basal membrane to the forming yolk membrane. To identify the nature of these junctions, they performed a parental RNAi screen and found that the disruption of Innexin7a (Inx7a), whose Drosophila orthologue is dispensable for cellularisation, significantly impairs basal cell closure during Tribolium castaneum cellularisation. Inx7a is localised at the basal membrane of the forming epithelium and is required for its formation and for the stabilisation of the invaginated membrane. This study provides insight into the mechanisms of cellularisation in a non-Drosophila insect model, which are likely to be conserved in a greater number of insects.
PLUS:
An interview with Deepak Srivastava
 Deepak Srivastava is a Director at the Gladstone Institute of Cardiovascular Disease and a Distinguished Professor in Paediatric Developmental Cardiology at the University of California, San Francisco. As well as caring for sick children as a physician at the Benioff Children’s Hospital in San Francisco, he runs an active research group that studies the biology of heart development and regeneration. In March 2015, we met up with Deepak and asked him about his career. See the Spotlight article on p. 2083
Deepak Srivastava is a Director at the Gladstone Institute of Cardiovascular Disease and a Distinguished Professor in Paediatric Developmental Cardiology at the University of California, San Francisco. As well as caring for sick children as a physician at the Benioff Children’s Hospital in San Francisco, he runs an active research group that studies the biology of heart development and regeneration. In March 2015, we met up with Deepak and asked him about his career. See the Spotlight article on p. 2083
An interview with Rudolf Jaenisch
 Rudolf Jaenisch is a Professor of Biology at Massachusetts Institute of Technology, a founding member of the Whitehead Institute for Biomedical Research and the current president of the International Society for Stem Cell Research (ISSCR). In recognition of his pioneering research, he recently received the 2015 March of Dimes Prize in Developmental Biology. At the recent Keystone Meeting on ‘Transcriptional and Epigenetic Influences on Stem Cell States’ in Colorado, we had the opportunity to talk to him about his life and work. See the Spotlight article on p. 2085
Rudolf Jaenisch is a Professor of Biology at Massachusetts Institute of Technology, a founding member of the Whitehead Institute for Biomedical Research and the current president of the International Society for Stem Cell Research (ISSCR). In recognition of his pioneering research, he recently received the 2015 March of Dimes Prize in Developmental Biology. At the recent Keystone Meeting on ‘Transcriptional and Epigenetic Influences on Stem Cell States’ in Colorado, we had the opportunity to talk to him about his life and work. See the Spotlight article on p. 2085
Neuronal polarization
 Neurons are highly polarized cells with structurally and functionally distinct processes called axons and dendrites. This polarization, which underlies the directional flow of information in the central nervous system, is crucial for correct development and function. This short review and accompanying poster highlight recent advances in this fascinating field, with an emphasis on the signaling mechanisms underlying axon and dendrite specification in vitro and in vivo. See the Development at a Glance article on p. 2088
Neurons are highly polarized cells with structurally and functionally distinct processes called axons and dendrites. This polarization, which underlies the directional flow of information in the central nervous system, is crucial for correct development and function. This short review and accompanying poster highlight recent advances in this fascinating field, with an emphasis on the signaling mechanisms underlying axon and dendrite specification in vitro and in vivo. See the Development at a Glance article on p. 2088
Orchestrating liver development
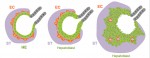 The liver is a central regulator of metabolism, and liver failure thus constitutes a major health burden. Understanding how this complex organ develops during embryogenesis will yield insights into how liver regeneration can be promoted and how functional liver replacement tissue can be engineered. Here, Gordillo, Evans and Gouon-Evans review the lineage relationships, signaling pathways and transcriptional programs that orchestrate hepatogenesis. See the Review on p. 2094
The liver is a central regulator of metabolism, and liver failure thus constitutes a major health burden. Understanding how this complex organ develops during embryogenesis will yield insights into how liver regeneration can be promoted and how functional liver replacement tissue can be engineered. Here, Gordillo, Evans and Gouon-Evans review the lineage relationships, signaling pathways and transcriptional programs that orchestrate hepatogenesis. See the Review on p. 2094
Adding a spatial dimension to postnatal ventricular-subventricular zone neurogenesis
 The neural stem cells (NSCs) located in the largest germinal region of the forebrain, the ventricular-subventricular zone (V-SVZ), replenish olfactory neurons throughout life. However, V-SVZ NSCs are heterogeneous: they have different embryonic origins and give rise to distinct neuronal subtypes depending on their location. In this Review, we discuss how this spatial heterogeneity arises, how it affects NSC biology, and why its consideration in future studies is crucial for understanding general principles guiding NSC self-renewal, differentiation and specification. See the Review on p. 2109
The neural stem cells (NSCs) located in the largest germinal region of the forebrain, the ventricular-subventricular zone (V-SVZ), replenish olfactory neurons throughout life. However, V-SVZ NSCs are heterogeneous: they have different embryonic origins and give rise to distinct neuronal subtypes depending on their location. In this Review, we discuss how this spatial heterogeneity arises, how it affects NSC biology, and why its consideration in future studies is crucial for understanding general principles guiding NSC self-renewal, differentiation and specification. See the Review on p. 2109


 (No Ratings Yet)
(No Ratings Yet)