In Development this week (Vol. 142, Issue 14)
Posted by Seema Grewal, on 21 July 2015
Here are the highlights from the current issue of Development:
HIF1α muscles in on regeneration

Top Notch insights into differential signalling

The two closely related mammalian Notch receptors Notch1 and Notch2 have been shown to play different, and sometimes opposing, roles in development and disease. But what is the mechanistic basis of these differences? Here, Raphael Kopan and colleagues address this question using mice in which the intracellular domains (ICDs) of these two Notch receptors have been swapped (p. 2452). They first show that ICD swapping has little effect on the development of organs in which either Notch1 (T cells, skin, the inner ear and endocardium) or Notch2 (the liver, eye, cardiac neural crest and lung) is known to act alone or is dominant over it paralogue, suggesting that the ICDs are interchangeable. In the case of Notch dosage-sensitive tissues, the researchers further show that the phenotypes observed are due to haploinsufficiency and not due to ICD composition. Together, these and other findings lead the authors to conclude that both the strength of Notch signalling (defined by the number of ICD molecules that get cleaved from the receptor and reach the nucleus) and the duration of signalling (the half-life of active ICD complexes) contribute to the differences between Notch1 and Notch2 functions in many developmental contexts.
An extended view of musculoskeletal development

The musculoskeletal system is made up of a number of tissue types, including bone, muscle, tendon and cartilage. While the development of each of these tissues has been studied, how they integrate into a functional superstructure, and the extent to which they develop independently, is unclear. Now, Ronen Schweitzer and co-workers investigate this interdependency by analysing tendon development in mice that have defective muscle or cartilage developmental programmes (p. 2431). They report that whereas tendon development in the zeugopod (arm/leg) is dependent on muscle, autopod (paw) tendon development occurs independently of muscle and instead requires cues from skeletal tissues. These findings suggest that autopod and zeugopod tendon segments can develop independently and, in line with this, the researchers demonstrate that they are derived from distinct progenitor pools. They further show that tendons are integrated in a modular fashion, whereby zeugopod muscles first connect to their respective autopod tendon via an anlagen of tendon progenitors in the presumptive wrist and the tendons then elongate proximally in parallel with skeletal growth. Based on their findings, the authors put forward a novel integrated model for limb tendon development.
Fishing for clues into tooth replacement

Unlike mice and humans, basal vertebrates such as sharks and fish exhibit continuous tooth renewal and thus offer an attractive model for studying tooth replacement. Here, by taking advantage of the natural variation in threespine stickleback fish populations, Craig Miller and colleagues examine the genetic and developmental basis of tooth regeneration (p. 2442). They first compare the tooth morphology of three laboratory-reared populations: one marine population and two freshwater populations. They report that, relative to the ancestral marine population, the two freshwater populations exhibit increased numbers of pharyngeal teeth, increased tooth plate areas and decreased intertooth spacing. The increase in tooth number, they demonstrate, occurs late in development and is due to an elevated rate of tooth replacement. When comparing the two freshwater populations, the researchers further note that the spatial patterning of newly formed teeth and the timing of their emergence differ between the two populations, suggesting that they use distinct developmental mechanisms. Finally, using quantitative trait loci mapping, the researchers show that different genomic regions contribute to the increase in tooth number in the two freshwater populations. These findings support a model for convergent evolution via distinct developmental routes and provide insights into the genetic factors that govern tooth replacement.
PLUS:
An interview with Brigid Hogan
 We recently interviewed Brigid Hogan, a developmental biologist who has worked extensively on the early stages of mouse development and is now unravelling the mysteries of lung organogenesis. She is the George Barth Geller Professor and Chair of the Department of Cell Biology at Duke University Medical Center. Brigid is also the winner of the 2015 Society for Developmental Biology (SDB) Lifetime Achievement Award. See the Spotlight article on p. 2389
We recently interviewed Brigid Hogan, a developmental biologist who has worked extensively on the early stages of mouse development and is now unravelling the mysteries of lung organogenesis. She is the George Barth Geller Professor and Chair of the Department of Cell Biology at Duke University Medical Center. Brigid is also the winner of the 2015 Society for Developmental Biology (SDB) Lifetime Achievement Award. See the Spotlight article on p. 2389
The retromer complex in development and disease
 The retromer complex is a multimeric protein complex involved in recycling proteins from endosomes to the trans-Golgi network or plasma membrane. Here, Wang and Bellen summarise the role of the retromer complex in developmental processes, neuronal maintenance, and human neurodegenerative diseases. See the Development at a Glance article on p. 2392
The retromer complex is a multimeric protein complex involved in recycling proteins from endosomes to the trans-Golgi network or plasma membrane. Here, Wang and Bellen summarise the role of the retromer complex in developmental processes, neuronal maintenance, and human neurodegenerative diseases. See the Development at a Glance article on p. 2392
LIN28: roles and regulation in development and beyond
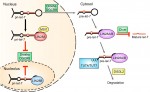 LIN28 is an RNA-binding protein best known for its roles in promoting pluripotency via regulation of the microRNA let-7. However, recent studies have uncovered new roles for LIN28, suggesting that it is more than just a regulator of miRNA biogenesis. Here, Tsialikas and Romer-Seibert review how LIN28 functions in development and disease. See the Primer on p. 2397
LIN28 is an RNA-binding protein best known for its roles in promoting pluripotency via regulation of the microRNA let-7. However, recent studies have uncovered new roles for LIN28, suggesting that it is more than just a regulator of miRNA biogenesis. Here, Tsialikas and Romer-Seibert review how LIN28 functions in development and disease. See the Primer on p. 2397


 (1 votes)
(1 votes)