In Development this week (Vol. 142, Issue 16)
Posted by Seema Grewal, on 18 August 2015
Here are the highlights from the current issue of Development:
Probing gene expression dynamics in stem cells
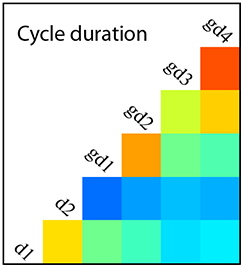 The accurate control of gene expression is essential for cell differentiation during development but how do heterogeneous and fluctuating gene expression levels influence cell fate choices? Here (p. 2840), using a novel quantitative and high-content imaging platform, Jonathon Chubb and colleagues investigate how various cell- and population-based features are coupled to Nanog reporter expression in mouse embryonic stem cells (ESCs). They first show that cell cycle times are heterogeneous within ESCs but correlate with Nanog reporter expression; low expression levels are found in both long and short cell cycles but reporter expression tends to be highest in longer cycles. The transition to ground-state pluripotency (triggered by 2i treatment), they report, correlates with longer and more variable cell cycle times. Looking at lineage history, the researchers further reveal that all cells within a lineage are strongly related with regards to both cell cycle times and reporter expression. Modelling further suggests that some element of the cell environment plays a role in stabilising gene expression between generations. Finally, the researchers highlight a correlation between cell density and both cell cycle behaviour and reporter gene expression. Based on these and other findings, the authors propose that simple deterministic views of stem cell states need rethinking.
The accurate control of gene expression is essential for cell differentiation during development but how do heterogeneous and fluctuating gene expression levels influence cell fate choices? Here (p. 2840), using a novel quantitative and high-content imaging platform, Jonathon Chubb and colleagues investigate how various cell- and population-based features are coupled to Nanog reporter expression in mouse embryonic stem cells (ESCs). They first show that cell cycle times are heterogeneous within ESCs but correlate with Nanog reporter expression; low expression levels are found in both long and short cell cycles but reporter expression tends to be highest in longer cycles. The transition to ground-state pluripotency (triggered by 2i treatment), they report, correlates with longer and more variable cell cycle times. Looking at lineage history, the researchers further reveal that all cells within a lineage are strongly related with regards to both cell cycle times and reporter expression. Modelling further suggests that some element of the cell environment plays a role in stabilising gene expression between generations. Finally, the researchers highlight a correlation between cell density and both cell cycle behaviour and reporter gene expression. Based on these and other findings, the authors propose that simple deterministic views of stem cell states need rethinking.
Fattening up neural development
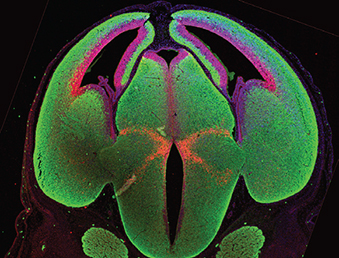 Fat family atypical cadherins are known to regulate planar cell polarity and growth control in Drosophila. In mammals, four Fat genes (Fat1 to Fat4) have been identified but relatively little is known about how these regulate mammalian embryogenesis. Here, Helen McNeill and co-workers identify a role for Fat proteins in regulating various aspects of brain development in mice (p. 2781). Using mutant mice, the researchers first identify a role for Fat1 in neural tube closure; Fat1mutants display cranial neural tube closure defects leading to exencephaly. They further show that the cortex of these mutant embryos exhibits elongated ventricles, linked to an increase in radial precursor cell proliferation. Accordingly, the knockdown of Fat1 by in utero electroporation in the developing cortex causes an increase in radial precursor cell proliferation and perturbs neuronal differentiation and migration. The researchers further show that Fat4 interacts genetically with Fat1 to control these processes. Finally, they reveal that Fat1 and Fat4 bind to distinct sets of actin regulators and apical junction proteins, respectively. Together, these findings lead the authors to propose a model in which Fat1-Fat4 dimer formation brings together diverse proteins at apical junctions to regulate both apical constriction and progenitor cell divisions in the neural tube.
Fat family atypical cadherins are known to regulate planar cell polarity and growth control in Drosophila. In mammals, four Fat genes (Fat1 to Fat4) have been identified but relatively little is known about how these regulate mammalian embryogenesis. Here, Helen McNeill and co-workers identify a role for Fat proteins in regulating various aspects of brain development in mice (p. 2781). Using mutant mice, the researchers first identify a role for Fat1 in neural tube closure; Fat1mutants display cranial neural tube closure defects leading to exencephaly. They further show that the cortex of these mutant embryos exhibits elongated ventricles, linked to an increase in radial precursor cell proliferation. Accordingly, the knockdown of Fat1 by in utero electroporation in the developing cortex causes an increase in radial precursor cell proliferation and perturbs neuronal differentiation and migration. The researchers further show that Fat4 interacts genetically with Fat1 to control these processes. Finally, they reveal that Fat1 and Fat4 bind to distinct sets of actin regulators and apical junction proteins, respectively. Together, these findings lead the authors to propose a model in which Fat1-Fat4 dimer formation brings together diverse proteins at apical junctions to regulate both apical constriction and progenitor cell divisions in the neural tube.
Tissue regeneration: from Hippo to flies and fish
The Hippo pathway, best known for its role in growth control, has been implicated in tissue repair and regeneration in various contexts. In this issue, two papers provide insights into how Hippo signalling regulates tissue growth during regeneration.
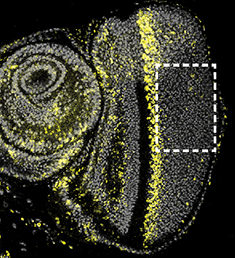 In the first report (p. 2740), Joy Meserve and Robert Duronio study the Drosophila eye to investigate the mechanisms that allow quiescent cells to re-enter the cell cycle and proliferate in response to tissue damage. Using an RNAi screen, they reveal that scalloped (sd), which encodes a transcriptional effector of the Hippo pathway, is required for compensatory proliferation following tissue damage. They demonstrate that Sd and its binding partner Yorkie (Yki) are required to induce Cyclin E expression and hence drive S-phase entry in regenerating eye discs. The researchers further show that Ajuba (Jub), an upstream regulator of Hippo signalling, is needed for cell cycle re-entry. Given the roles of Jub in sensing epithelial integrity, the authors propose that the apoptotic force induced by tissue damage in this context triggers Jub and Sd/Yki activation that, in turn, allows for compensatory proliferation and tissue repair.
In the first report (p. 2740), Joy Meserve and Robert Duronio study the Drosophila eye to investigate the mechanisms that allow quiescent cells to re-enter the cell cycle and proliferate in response to tissue damage. Using an RNAi screen, they reveal that scalloped (sd), which encodes a transcriptional effector of the Hippo pathway, is required for compensatory proliferation following tissue damage. They demonstrate that Sd and its binding partner Yorkie (Yki) are required to induce Cyclin E expression and hence drive S-phase entry in regenerating eye discs. The researchers further show that Ajuba (Jub), an upstream regulator of Hippo signalling, is needed for cell cycle re-entry. Given the roles of Jub in sensing epithelial integrity, the authors propose that the apoptotic force induced by tissue damage in this context triggers Jub and Sd/Yki activation that, in turn, allows for compensatory proliferation and tissue repair.
 In a second paper, Antonio Jacinto and colleagues reveal a role for the Hippo pathway effector Yap in zebrafish fin regeneration (p. 2752). Fin regeneration involves three steps – wound healing, blastema formation and tissue outgrowth – and the researchers show that Yap activation (and hence nuclear localisation) is dynamic during these steps. Yap is nuclear during wound healing, remains nuclear during blastema formation, and then is cytoplasmic in regions distal to the wound but nuclear in proximal regions during outgrowth. They further show, by modulating Yap levels, that Yap regulates cell proliferation and the expression of key regeneration factors. The researchers also report that Yap localisation correlates with changes in cell density and cell morphology along the blastema proximal-distal axis. Finally, they observe similar gradients in α-catenin and F-actin localisation, suggesting a model in which a mechanotransduction process involving changes in cell morphology, junctional assembly and the cytoskeleton controls the activation of Yap to regulate tissue regeneration.
In a second paper, Antonio Jacinto and colleagues reveal a role for the Hippo pathway effector Yap in zebrafish fin regeneration (p. 2752). Fin regeneration involves three steps – wound healing, blastema formation and tissue outgrowth – and the researchers show that Yap activation (and hence nuclear localisation) is dynamic during these steps. Yap is nuclear during wound healing, remains nuclear during blastema formation, and then is cytoplasmic in regions distal to the wound but nuclear in proximal regions during outgrowth. They further show, by modulating Yap levels, that Yap regulates cell proliferation and the expression of key regeneration factors. The researchers also report that Yap localisation correlates with changes in cell density and cell morphology along the blastema proximal-distal axis. Finally, they observe similar gradients in α-catenin and F-actin localisation, suggesting a model in which a mechanotransduction process involving changes in cell morphology, junctional assembly and the cytoskeleton controls the activation of Yap to regulate tissue regeneration.
PLUS:
 An interview with Caroline Dean
An interview with Caroline Dean
Caroline Dean is a plant biologist based at the John Innes Centre in Norwich, UK, who works on the epigenetic mechanisms that regulate vernalisation. We talked to Caroline about her career, her FEBS EMBO Women in Science Award, and her thoughts on the plant field. See the Spotlight article on p. 2725
The embryo reunited with its membranes in Göttingen
In this meeting review, Claudio Stern summarises the work and advances presented at the recent EMBO Workshop ‘Embryonic-Extraembryonic Interfaces’, which took place in Germany. See the Meeting Review on p. 2727
Primordial germ cells: the first cell lineage or the last cells standing?
In this Hypothesis article, Johnson and Alberio propose that the determinative mechanisms for PGC specification in most model systems evolved to promote speciation and evolvability, not to maintain the germ line. See the Hypothesis on p. 2730



 (No Ratings Yet)
(No Ratings Yet)