In Development this week (Vol. 143, Issue 5)
Posted by Seema Grewal, on 1 March 2016
Here are the highlights from the current issue of Development:
Mak(or)in’ the switch to adulthood

The juvenile-to-adult (J/A) transition of many animals, from worms to humans, is regulated by the highly conserved RNA-binding protein LIN-28. In this and other contexts – including stem cell renewal versus differentiation decisions – LIN-28 acts to suppress the production of the microRNA let-7, which in turn inhibits a suite of downstream genes, most notably the translational regulator LIN-41. Now, using the J/A transition of C. elegans as a model, David Fitch and colleagues (799) identify a new player in this axis, the Makorin orthologue LEP-2. lep-2 mutant adults display a number of juvenile characteristics, including failure of male tail tip retraction, continued moulting into adulthood and defective male sexual behaviour. The authors provide evidence that LEP-2 acts to promote degradation of LIN-28 in larval stages, which is necessary for the J/A transition. The underlying molecular mechanism has yet to be resolved: as a putative E3 ubiquitin ligase, LEP-2 might directly target LIN-28 for degradation or it may act indirectly. Given the conservation of the Makorin family, along with data implicating mammalian Makorins in cell state transitions and in the timing of puberty onset, it is possible that this Makorin/LIN-28 interaction could control developmental switches in multiple contexts.
Maintaining and reprogramming sexual identity

Gonadal sexual identity is determined during development, but must also be maintained in adulthood. In Drosophila, the transcription factor Chinmo has been identified as a key regulator of male identity in the adult testis: it promotes expression of the male sex determinant DsxM (the homologue of which, DMRT1, is a key regulator of male identity in mouse testes). In chinmo mutants, somatic stem cells of the adult testis adopt female fate. Now (754), Erika Matunis and co-workers show that Chinmo is not only necessary but also sufficient to promote male fate. Overexpression of chinmo in adult ovarian somatic cells leads to severe oogenesis phenotypes. Marker expression analysis suggests thatchinmo-overexpressing somatic cells lose female identity and gain male fate. Strikingly, this also appears to affect the sexual fate of the germ cells: a proportion of ovary germ cells start to express male markers upon somatic chinmo expression. Unlike in testis, Chinmo in the ovary does not appear to promote DsxM expression, implying that there must be alternative mechanisms for masculinisation of somatic cells. Although these mechanisms have yet to be uncovered, these data provide strong evidence that sexual identity of the Drosophila adult ovary can be reprogrammed, and that sexual fate must be actively maintained throughout life.
At the heart of histone methylation

Mutations in the histone methyltransferase KMT2D are associated with Kabuki Syndrome – a haploinsufficient congenital, multi-organ syndrome that frequently includes severe heart defects. However, the role of KMT2D in the heart has not been analysed in detail. Benoit Bruneau and colleagues now address this (810), generating several conditional Kmt2d mouse mutants and analysing them both phenotypically and using genome-wide approaches. They find that cardiac deletion of Kmt2d causes embryonic lethality with defects in heart morphology and cardiomyocyte proliferation. Global gene expression analysis demonstrates dysregulation of genes associated with cell cycle regulation, ion homeostasis and hypoxia signalling. Functionally, ventricular calcium handling appears impaired. KMT2D is involved in H3K4 mono- and di-methylation; consistent with this, ChIP-Seq data demonstrate that Kmt2d depletion causes loss of H3K4me1 and me2 at specific loci. By correlating these data with the RNA-seq profiles and ChIP-Exo data for KMT2D chromatin binding, the authors are able to identify a small number of high-confidence targets for KMT2D, functions of which are consistent with the phenotypes observed upon Kmt2d deletion. As well as shedding light on the important role of KMT2D in mouse heart development, these data may have implications for the aetiology of the heart defects observed in Kabuki syndrome.
TFAP2C: a key controller of placental growth

In mammals, proper placental development is essential for growth and viability of the embryo. The transcription factor TFAP2C is known to be important for specification and maintenance of trophoblast stem cells (placental progenitors), but whether this factor also plays roles at later stages of placental development is less well understood. On 787, Hubert Schorle and co-workers provide insights into the role of TFAP2C in a subset of placental progenitors, the TPBPA-expressing population that forms the junctional zone of the placenta. Loss of Tfap2c from this population leads to growth defects in the junctional zone, with reduced numbers of TPBPA+ cell-derived trophoblasts. Microarray analysis and follow-up experiments provide evidence that TFAP2C controls several key aspects of placental development: it inhibits Cdkn1a, a cell cycle inhibitor; promotes expression and activation of Akt to regulate glycogen synthesis; and promotes MAPK pathway activity – important for trophoblast proliferation and differentiation – by repressing the Dusp family of MAPK inhibitors. Importantly, this conditional mouse mutant provides a model for intrauterine growth retardation, as mutant embryos show lower foetal and birth weight. Preliminary data in a human trophoblast cell model suggests that this important role of TFAP2C may be conserved.
PLUS:
Building and re-building the heart by cardiomyocyte proliferation
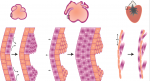 Dissecting the cellular and molecular mechanisms that promote cardiomyocyte proliferation throughout life, deciphering why proliferative capacity normally dissipates in adult mammals and deriving means to boost this capacity, are primary goals in cardiovascular research. Here, discuss the cellular and molecular mechanisms that control cardiomyocyte proliferation, during both heart development and regeneration across various species. See the Review article on p. 729
Dissecting the cellular and molecular mechanisms that promote cardiomyocyte proliferation throughout life, deciphering why proliferative capacity normally dissipates in adult mammals and deriving means to boost this capacity, are primary goals in cardiovascular research. Here, discuss the cellular and molecular mechanisms that control cardiomyocyte proliferation, during both heart development and regeneration across various species. See the Review article on p. 729
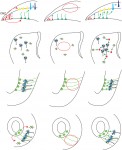
A comparative view of regenerative neurogenesis in vertebrates
summarize the striking similarities in the essential molecular and cellular properties of adult neural stem cells between different vertebrate species, both under physiological and reparative conditions. They also discuss differences in the reparative process across evolution and how the study of non-mammalian models can provide insights into both basic neural stem cell properties and stimulatory cues shared between vertebrates. See the Review on p. 741
Featured movie
This movie shows the mating behaviour of wild type male C. elegans. This behaviour is disrupted in mutants of lep-2, a new heterochronic gene identified by Fitch and colleagues. Read the paper here.


 Alessandro Alunni and
Alessandro Alunni and (No Ratings Yet)
(No Ratings Yet)