A Day in the Life of a Bat Lab
Posted by AaronHarnsberger, on 15 March 2016
I am Aaron Harnsberger, a second year Master’s degree student in the Department of Biological Sciences at Idaho State University in Pocatello, Idaho. The focus of this lab is on genetic regulatory divergence that results in the diversity of mammalian morphologies. These morphological differences can be observed at various stages of development. In this lab we use bats as our model organism. There are no other members working in this lab besides myself. There are a growing number of labs throughout the world that also use bats to study evolutionary and developmental biology. The species of bat that I use is Carollia perspicillata (C. perspicillata) or simply Carollia (Fig. 1). On average an adult Carollia bat is about 19 grams (Fig. 2). Although it is possible to maintain Carollia as a laboratory bat (Rasweiler et al., 2009), we do not have a bat colony in the lab so before I can conduct experiments I make trips to the field to collect animals from the wild.

At this point, you might be asking, “Why use bats?”. Bats have some very interesting adaptations that make them a great model for studying evolutionary development. They are the only mammal to achieve powered flight. The development of wings where other mammals have paws, fins, hooves or hands is one adaptation that I find fascinating. Another very interesting adaptation discovered in bats is delayed development. This is when a pregnant female delays development of the embryo. In other mammalian species this typically occurs at the blastocyst stage before implantation in the uterus. However, in Carollia delayed development occurs after implantation at the gastrulation stages (Rasweiler et al., 1997). This delay may be highly synchronous in colonies, and cues that initiate the delay and end it are still unknown. Bats can have a long life span when compared to other mammals of similar size. In addition, bats may respond differently to injury and the healing process may lack inflammation. There are more features, but as you can see bats are unique models for many biological questions.

Back to the field, which in this case, is the country of Trinidad and Tobago (Fig. 3). These field collection trips to the island of Trinidad require careful planning and weeks of time to catch bats and collect embryos. I do not catch the bats on these trips by myself. The ‘Bat Team’ this year consisted of John Rasweiler, Richard Behringer, Simeon (Patsy) Williams, Joseph Truechen and myself. Patsy and Joseph are local field assistants who help us find and collect the bats. The ‘Bat Team’ has consisted of many other biologists that have made this collection trip throughout the years, starting with the initial trip in 2000.
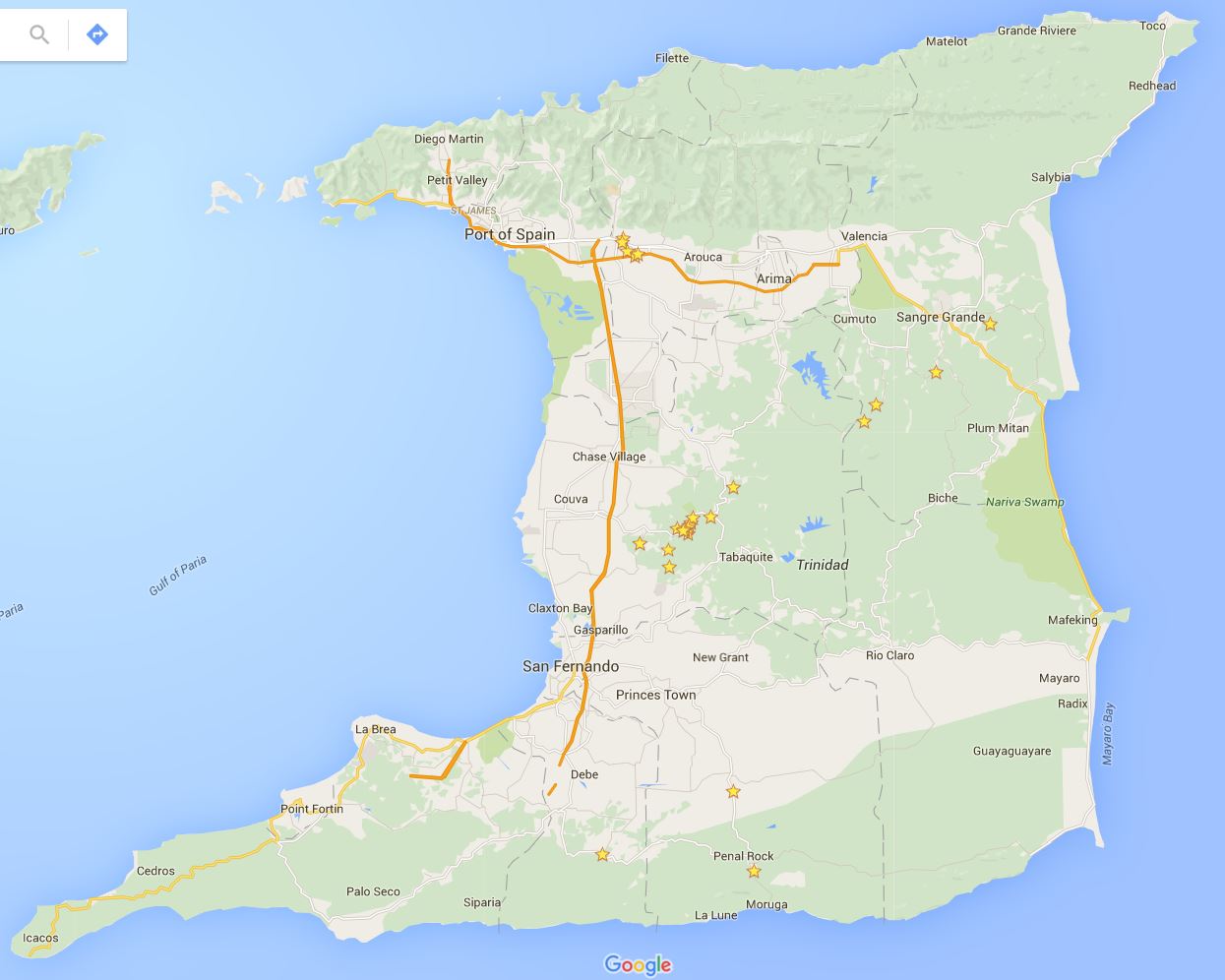
First and foremost, a collection permit through the Wildlife Section of the Division of Forestry of Trinidad and Tobago is required. We give them a specific number of bats we would like to collect and plan our trip according to this number. Next we need a place to process our samples. This is the Department of Life Sciences at the University of West Indies (UWI) in St. Augustine that has lab space, microscopes and other facilities. We also need lodging since the trips take several days. This is arranged through University Housing. Finally, we rent a car to take us into the deep forest that has enough room for several passengers and our gear. Once all of this is in order we can begin catching bats.

To catch bats, we have to travel into the northern or central areas of the island. We look for certain habitats that can lead us to specific roosting sites. To achieve this, we conduct scouting trips and spend many hours driving, parking, looking around, taking notes, and if all goes well catching bats.

We use lots of different gear to catch bats. We occasionally need ladders to get into some of the places that bats roost, for example abandoned concrete water tanks (Fig. 6). On the occasions that we do need a ladder we have to carry it in, sometimes through thick forest (Fig. 4). We use nets similar to butterfly nets to catch the bats, but much longer to reach high places where they roost (Fig. 6). After catching the bats, we sort through them, releasing males and checking females for age and stage of pregnancy using external visual appearances. We keep these female bats in a field box which allows for safe transport of the bats (Fig. 5). After a morning of collecting, we return to the lab at UWI with the bats to isolate the embryos. Once embryos are dissected we process them for various types of analyses, including morphological, histological and molecular studies. To bring the bat embryos back to the lab in Idaho, we obtain an exportation permit, also from the Wildlife Section of the Division of Forestry of Trinidad and Tobago.

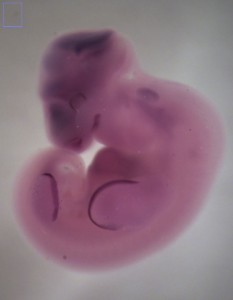
Now that I have the bat embryos to work with I can spend time in the lab back in Idaho conducting experiments. I use whole-mount in situ hybridization (WISH) to examine the spatial distribution of transcripts for specific genes at different stages of development. Using the Carollia embryo staging techniques (Cretekos, et al. 2005), I can identify embryos at specific stages of development. This gives me specific time points to look for gene expression at critical developmental stages (Fig. 7). Many times these expression patterns are similar yet different from those in the mouse.
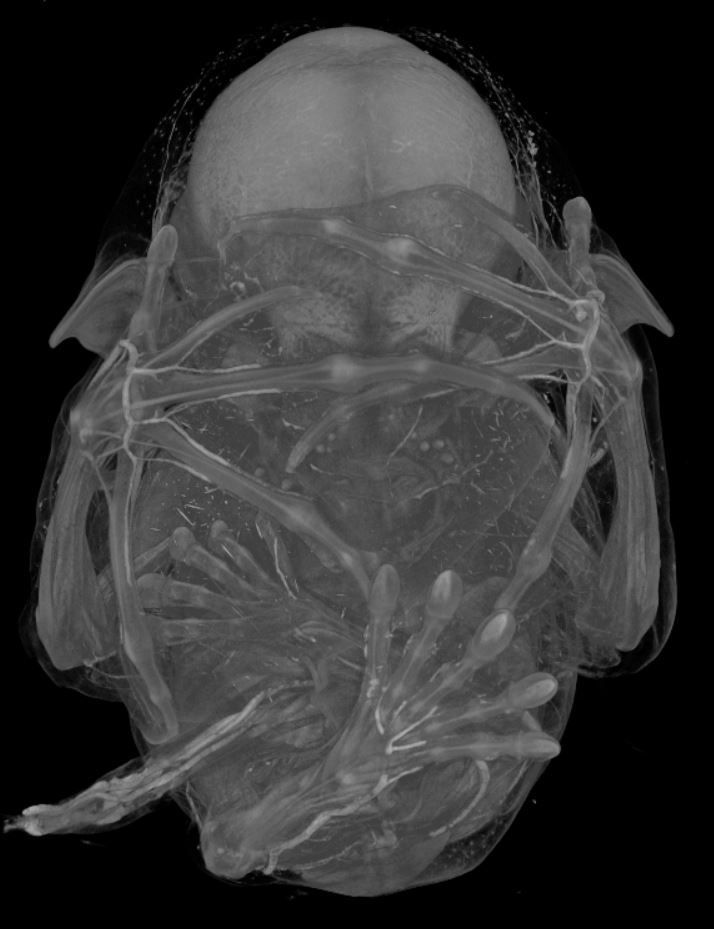
I have also used micro computed tomography (uCT) to visualize the tissue anatomy of bat embryos (Fig. 8). A uCT scan is a high resolution x-ray that is used to make a three-dimensional image. The differences in contrast allow me to identify different tissue types in the bat embryos. Again, taking advantage of the Carollia staging system, I have uCT scanned stages of development in C. perspicillata to examine particular tissues of interest for my project. The data collection was completed in a few weeks’ time. The image processing and analysis of this data has been an ongoing project in the lab.
As you can see a day in the life of a bat lab is not usually just a day, and some of the work may not be typical in other developmental biology labs (Fig 9).

References
Cretekos, C. J., Weatherbee, S. D., Chen, C., Badwaik, N. K., Niswander, L., Behringer, R. R. & Rasweiler, J. J. IV. (2005). Embryonic staging system for the short-tailed fruit bat, Carollia perspicillata, a model organism for the mammalian order Chiroptera, based upon timed pregnancies in captive-bred animals. Developmental Dynamics, 233: 721-738.
Rasweiler, J. J. IV & Badwaik, N. K. (1997). Delayed development in the short-tailed fruit bat, Carollia perspicillata. Journal of Reproductive Fertility, 109(1): 7-20.
Rasweiler, J. J. IV, Cretekos, C. J. & Behringer R. R. (2009). The short-tailed fruit bat Carollia perspicillata: a model for studies in reproduction and development. Cold Spring Harbor Protocols, 2009(3): pdb.emo118.
Tags: Bat, Carollia, WISH, in situ, uCT, microCT, development, ISU, Idaho State University
 This post is part of a series on a day in the life of developmental biology labs working on different model organisms. You can read the introduction to the series here and read other posts in this series here.
This post is part of a series on a day in the life of developmental biology labs working on different model organisms. You can read the introduction to the series here and read other posts in this series here.


 (9 votes)
(9 votes)