Back to basics: Unraveling the mystery of mammalian germ cell connectivity using lineage analysis
Posted by Lei L, on 9 May 2016
Lei Lei and Allan Spradling
Germ cells are unique among all metazoan cells in their ability to persist from one generation to the next. In seeking to understand how germ cells acquire and maintain immortality, a logical place to start with is the distinctive cell biological properties these cells display. For example, in many organisms, developing germ cells socially interact just before and during meiosis. In Drosophila, a founder germ cell called a cystoblast synchronously divides four times without undergoing cytokinesis to produce sixteen interconnected cells known as a germline cyst. Interconnected germ cells within ovarian cysts transport cytoplasm, organelles and RNAs to one germ cell that will survive and develop as oocyte. The rest of the fifteen sister germ cells that transfer materials, undergo apoptosis and are called nurse cells (de Cuevas et al., 1997).
Whether mammals also nurse developing oocytes has remained unclear. In fetal mammalian ovaries, germ cells appear morphologically similar, rather than as recognizable oocytes and nurse cells. After migrating to the fetal ovary, primordial germ cells (PGCs) proliferate and the resulting cells either differentiate into primary oocytes or undergo apoptosis. In humans, PGCs proliferate into about 7 million germ cells at 20 weeks of gestation and these give rise to 1 million primary oocytes at birth (Baker, 1963). In mice, PGCs generate about 20,000 germ cells at embryonic day 14.5 (E14.5) that differentiate into about 4,000 primary oocytes in the postnatal day 4 (P4) ovary (Lei and Spradling, 2013). These germ cell losses could be explained if the distinction between nurse cell and oocyte was not difficult to recognize in mammals. In that case, the cells undergoing apoptosis would be nurse cells that had finished transferring important materials to the oocytes.
If mammalian fetal ovaries do contain nurse cells as well as differentiating oocytes, they should be interconnected in germline cysts, like in Drosophila and many other species. Efforts to characterize mouse germline cysts began in our group in 1995 when Dr. Melissa Pepling joined the laboratory. Pepling systematically studied intercellular bridges and nest development within the fetal ovary. She found evidence that cysts form based on an increasing number of intercellular bridges, and observed multiple bridges per cell, bridges containing microtubules and with luminal mitochondria apparently moving between sister germ cells. Strikingly, she documented that synchronous germ cell mitotic divisions occur at this time, initially in groups corresponding to powers of two. These observations strengthened the case that germline cysts form between E10.5 when PGCs reach the gonad, and E14.5, when germ cells cease mitosis and enter meiosis (Pepling and Spradling, 1998). A few years later, Pepling demonstrated that the breakdown of mouse cysts by apoptosis correlated with the formation of primary oocytes and primordial follicles (Pepling and Spradling, 2001). However, it was not possible to precisely describe the structure of the starting cysts or how they broke down into oocytes. How many oocytes derived from an initial cyst remained unknown.
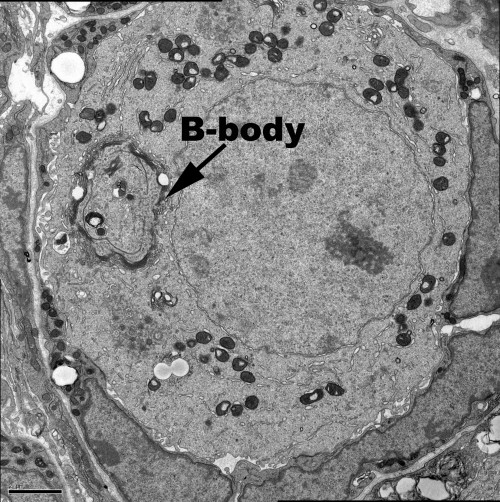
Oocytes from diverse species contain a visible mass of organelles known as the Balbiani body or mitochondrial cloud at the time they are first enclosed within a follicle. Specific mRNAs that later localize within the oocyte, including in the germ plasm, associate with the Xenopus Balbiani body (Kloc and Etkin, 1995). Up until this time, mouse oocytes were reported to be symmetrically organized and to lack a Balbiani body. However, studies in Drosophila showed that the Balbiani body is built mostly from organelles that are transferred from nurse cells into the oocyte around the time of follicle formation (Cox and Spradling, 2003, 2006). This prompted Pepling et al. (2007) to reinvestigate and show that a Balbiani body does form in mouse oocytes around the time of birth (Figure 1). The existence of a mouse Balbiani body raised the question of whether it forms like in Drosophila, by the transfer of its organelles and other components from connected nurse cells.
This was the situation when Lei Lei, a new postdoc with extensive previous experience in studying the mouse ovary, joined the lab in 2009. Clearly, the major roadblock to analyzing the behavior and function of cysts in the mouse ovary was simply the difficulty in recognizing individual cysts and their component germ cells. Since cyst cells share a common lineage, lineage-tracing methods offered a solution. Lei began to experiment with labeling only about one PGC per ovary at E10.5 when individual PGCs arrive at the fetal gonad using a general CreER-loxP system and low doses of Tamoxifen that rarely remove a blocker segment and activate yellow fluorescent protein (YFP) production. This approach, dubbed “single-cell lineage labeling,” would render the cells making up at least one cyst unambiguous in each ovary (Figure 2). Of course, using a low dose of Tamoxifen could not actually guarantee that exactly one PGC would be labeled. Some ovaries would have zero and a few would have two. However, even when two were labeled, they would usually be widely separated in the ovary, ensuring that their progeny remained separate. Lei would come to realize that this simpler paradigm could act as a key to throw open the treasure chest of mammalian oogenesis.
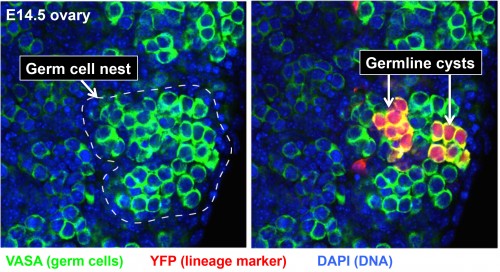
By analyzing the development of 164 individual marked E10.5 PGCs in this manner (about 500 are present per ovary), many longstanding questions were resolved (Lei and Spradling, 2013). All the PGCs follow the same developmental program, undergoing a similar number of divisions and producing a similar number of primary oocytes. PGCs start by generating germline cysts, since the initial number of labeled cells produced is always a power of two, reflecting the synchronous divisions first seen by Pepling. No PGCs from the fetal ovary remained undifferentiated, generated any other cell types or gave rise to oogonial stem cells, as previously postulated by some. Overall, between E10.5 and P4, 80% of PGC daughter cells underwent apoptosis, while the surviving 20% became primary oocytes. On average, each PGC produced an initial cyst that fragmented into about 5 derived cysts by E14.5 when mitotic division ceased. All germ cells then entered meiosis and generated about 6 primary oocytes by P4. The close agreement between the number of final derived cysts and the number of oocytes suggested that after an initial period of fragmentation, each remaining derived cyst generates one oocyte.
These experiments finally laid the groundwork for Lei to directly address the question of whether mouse cysts function like Drosophila cysts to nurse the oocyte and build the Balbiani body. First, she mapped the pattern of cellular interconnections within large, unfragmented cysts. In Drosophila cysts the oocyte always has 4 ring canals (intercellular bridges). Mouse germ cells with 3 or 4 ring canals were scattered throughout the early cysts, and this pattern may somehow presage how the initial cyst breaks into its derivatives. Lei went on to investigate whether organelles move between the interconnected cells within mouse cysts and accumulate in cells that become oocytes. She found that the Balbiani body could be used to distinguish cells transferring organelles (which lack a Balbiani body), from cells receiving organelles (which have a forming Balbiani body). By following centrosomes, mitochondria, Golgi elements and total cytoplasmic volume from E14.5 until all the cells have separated and either formed oocytes or died, she was able to prove that both organelles and cytoplasm are transferred non-randomly from the 80% of cells that later undergo apoptosis into the 20% that accumulate these materials, form a Balbiani body and become primary oocytes (Lei and Spradling, 2016). When cytoplasmic transport was blocked by inhibitors of microtubule polymerization or dynein-mediated movement, oocyte production and Balbiani body formation were greatly reduced. Concomitantly, apoptosis also declined, arguing that transfer is necessary to induce apoptosis. Thus, it is finally clear that fetal mammalian germ cells are divided into nurse cells and oocytes despite the fact that both enter meiosis and cannot easily be distinguished throughout much of fetal development by chromosomal or synaptonemal complex morphology.
During her final Spring and Summer in Baltimore, as this work was nearing completion, problems with the “Drosophila model” of transport through the ring canals became apparent. Mouse ring canals seemed to be important only during the early cyst stages. They shrink after E14.5, detach from the membrane joining adjacent germ cells, and disappear entirely after E19.5, well before oocyte differentiation is complete. The membranes separating germ cells develop large gaps at this same time, connecting the cells directly. The number of organelles and amount of cytoplasm moving into the oocytes is so large, it seemed more likely that they moved directly through membrane discontinuities rather than through the lumens of these small bridges. Moreover, confocal microscopy and EM studies began to make it clear that the germ cells undergoing apoptosis frequently have lost most of their cytoplasm, with little more than the nucleus being excluded from transfer. This suggested that germ cell apoptosis at these stages mostly destroyed nuclei, rather than entire cells. This realization should probably not have seemed so surprising. In Drosophila, the nurse cells in full-grown follicles synchronously transfer their cytoplasm into the oocyte just prior to entering apoptosis, a process known as “nurse cell dumping.” The nurse cell nuclei are excluded and show signs of apoptotic degeneration. Thus, the process of mouse oocyte formation by cytoplasmic dumping appears to be a variation on germ cell behaviors that are widespread among oocytes across many phyla.
These new insights require a revision of the traditional concept that mammalian oogenesis is highly inefficient. The largest single phase of germ cell loss, that occurring during oocyte differentiation, can now been seen as resulting from nurse cell apoptosis following the transfer of their cytoplasm and organelles to oocytes. Thus, “germ cell apoptosis” does not represent a waste of material, but rather a mechanism to start increasing the cytoplasmic/nuclear ratio of oocytes that will grow to be the largest cells in the mammalian body. The transfer of RNAs from nurse cells, including small RNAs, may also constitute an important part of the genome re-programming that takes place in the oocyte about this time.
The realization that mammalian oocytes develop in cysts and are supported by nurse cells provides new evidence that mammalian oocyte development is fundamentally similar to oogenesis in other animal groups. Indeed, even plant gametophytes transfer RNAs and other components between the somatic endosperm and the oocyte, in part to aid in the control of transposable elements (Creasey and Martienssen, 2010). Our work encourages the view that many of the unique properties of germ cells in all organisms, arise from fundamentally similar mechanisms that have been extensively conserved during evolution.
References:
Baker T.G. (1963). A quantitative and cytological study of germ cells in human ovaries. Proc. R. Soc. B. 150, 417-433.
Cox R.T. and Spradling A.C. (2003). A Balbiani body and the fusome mediate mitochondrial inheritance during Drosophila oogenesis. Development. 130,1579-1590.
Cox R.T. and Spradling A.C. (2006). Milton controls the early acquisition of mitochondria by Drosophila oocytes. Development. 133,3371-3377.
Creasey K.M. and Martienssen R.A. (2010). Germline reprogramming of heterochromatin in plants. Cold Spring Harb Symp Quant Biol. 75,269-274.
de Cuevas M., Lilly M.A. and Spradling A.C. (1997). Germline cyst formation in Drosophila. Annu. Rev. Genet. 31,405-28.
Kloc M. and Etkin L.D. (1995). Two distinct pathways for the localization of RNAs at the vegetal cortex in Xenopus oocytes. Development. 121,287-297.
Lei L. and Spradling A.C. (2013). Mouse primordial germ cells produce cysts that partially fragment prior to meiosis. Development. 140, 2075-2081.
Lei L. and Spradling A.C. (2016). Mouse oocytes differentiate through organelle enrichment from sister cyst germ cells. Science. 352,95-99.
Pepling M.E. and Spradling A.C. (1998). Female mouse germ cells form synchronously dividing cysts. Development. 125,3323-3328.
Pepling M.E. and Spradling A.C. (2001). Mouse ovarian germ cell cysts undergo programmed breakdown to from primordial follicles. Dev. Biol. 234, 339-351.
Pepling M. E., Wilhelm J.E. O’Hara A.L. Gephardt G.W. and Spradling A.C. (2007). Mouse oocytes within germ cell cysts and primordial follicles contain a Balbiani body. Proc Natl Acad Sci U S A. 104,187-192.


 (3 votes)
(3 votes)