In Development this week (Vol. 143, Issue 10)
Posted by Seema Grewal, on 17 May 2016
Here are the highlights from the current issue of Development:
How the zebrafish got its tail

Posterior axis elongation is a crucial process during metazoan development. In principle, axial extension can be driven either by tissue growth or by tissue rearrangement – or by a combination of the two. In vertebrates, studies based primarily on chick and mouse have suggested that the presence of a posterior proliferative zone is key for driving axial elongation. Here (p. 1732), Ben Steventon and colleagues assess the relative contribution of volumetric tissue growth versus tissue deformation (lengthening along one axis while narrowing in the other) to posterior body elongation in the zebrafish. During early phases, elongation proceeds without growth and with minimal cell proliferation. At later stages, cell proliferation is confined primarily to the segmented region of the embryo and not to the elongating unsegmented tailbud. Thus, convergent extension-like movements, involving cells entering the posterior trunk from more lateral positions, are the major driving force of elongation in the zebrafish. Through comparative analyses in other vertebrates, the authors’ data suggest a correlation between posterior volumetric growth – as seen in dogfish and mouse but not lamprey or zebrafish – and nutrient availability during embryogenesis.
Navigating the brain: the many paths of the interneurons

In the mouse forebrain, interneurons (INs) are born in the ganglionic eminences and preoptic area, and migrate from there to the cortex and other brain regions. The medial ganglionic eminence produces most INs of the neocortex – particularly the early born ones – whereas a minority of later-born cortical INs, as well as INs of other brain regions, derive from the caudal ganglionic eminence (CGE). What specifies the different populations of INs and how they reach their targets, is still incompletely understood. Michèle Studer and colleagues now (p. 1753) analyse IN migration from the CGE, finding that, in addition to the well-known caudal migratory stream (CMS), populations of INs also migrate rostrally by taking medial and lateral paths (the MMS and LMS, respectively). INs in the different paths appear to express different subsets of transcription factors and give rise to distinct sub-populations of mature INs. The authors identify the COUP-TFI nuclear receptor as a key regulator of identity and fate of the MMS and LMS INs. These data uncover an unexpected complexity in the migratory paths of CGE-derived INs that might be crucial for defining the huge diversity of mature IN types in the brain.
Head development under hypoxia

The early amniote embryo develops in a hypoxic environment: until blood circulation commences, oxygen availability in the developing tissues is low. What consequences does this have for development? On p. 1742, Nobue Itasaki and co-workers investigate the role of the hypoxia-responsive HIF pathway in regulating cranial neural crest cell (CNCC) production in the chick – which normally occurs in a hypoxic environment. They find that, upon embryo culture under normoxia, fewer CNCCs emerge from the neural tube; this phenotype can be rescued upon drug-induced activation of the HIF-1α pathway. Consistent with this, the authors observe reduced expression of known markers and regulators of migratory neural crest – several of which have previously been implicated as HIF-1α targets. It therefore appears that hypoxia is necessary for normal CNCC production, but is it sufficient to induce ectopic CNCCs? Activation of HIF-1α at later stages, after blood circulation has normally commenced, can induce CNCC production in in vitro explants, though only weak effects are seen in embryos. These data highlight the importance of the hypoxic environment for normal cranial development and imply that the initiation of blood circulation might have broad developmental consequences related to oxygen availability and HIF signalling.
Coactivator complexes in the spinal cord

In the vertebrate spinal cord, the LIM-homeodomain transcription factors Isl1 and Lhx3 act in concert with the linker protein NLI to regulate neuronal differentiation: Lhx3/NLI complexes promote V2a interneuron fate, whereas Lhx3/Isl1/NLI complexes direct motor neuron differentiation. However, how these complexes mediate transcriptional activation is still poorly understood. Soo-Kyung Lee and co-workers now (p. 1721) elucidate a role for the single-stranded DNA binding proteins Ssdp1/2 in this process. Ssdps are known to interact with NLI in various contexts and have been implicated as possible components of LIM-containing complexes. Here, the authors show that Ssdp1/2 interact with Lhx3, Isl1 and NLI, and that they are required for efficient activation of target genes – both in vivo and in vitro. In both chick and mouse, Ssdp1/2 knockdown compromises V2a and motor neuron differentiation. Mechanistically, Ssdp1/2 appear to be involved in recruiting histone-modifying enzymes to Lhx3/Isl1 targets, triggering deposition of active chromatin marks. Thus, this work identifies Ssdps as key components of transcriptional regulator complexes in the spinal cord and it is likely that they play similar roles in other developmental contexts.
Making a mammary gland with Blimp1

Uniquely among mammalian organs, the mammary gland of the female undergoes repeated cycles of expansion and regression during pregnancy, suckling and weaning. The tissue must therefore be equipped with hormone-responsive mechanisms for cell proliferation, differentiation and subsequent destruction. Various populations of progenitor cells have been identified and characterised, but our understanding of their properties and lineages is still incomplete. Here (p. 1663), Elizabeth Robertson and colleagues identify the transcriptional repressor Blimp1 as a marker of a progenitor population in the luminal compartment of the mouse mammary gland – rare in virgin females but expanding dramatically in pregnancy. They further demonstrate that Blimp1 is required for cell proliferation, polarity establishment and maintenance, and for the efficient production and secretion of milk components. While the mechanisms by which Blimp1 exerts these effects have yet to be elucidated, these data identify an important new regulator of mammary gland development and a new subpopulation of progenitor cells in the mammary epithelium.
PLUS:
Of sex and determination: marking 25 years of Randy, the sex-reversed mouse
 On Thursday 9 May 1991, the world awoke to front-page news of a breakthrough in biological research. From Washington to Wollongong, newspapers, radio and TV were abuzz with the story of a transgenic mouse in London called Randy. Why was this mouse so special? The mouse in question was a chromosomal female (XX) made male by the presence of a transgene containing the Y chromosome gene Sry. This sex-reversal provided clear experimental proof that Sry was the elusive mammalian sex-determining gene. Twenty-five years on, Koopman, Sinclair and Lovell-Badge reflect on what this discovery meant for our understanding of how males and females arise and what remains to be understood. Read the Spotlight on p. 1633
On Thursday 9 May 1991, the world awoke to front-page news of a breakthrough in biological research. From Washington to Wollongong, newspapers, radio and TV were abuzz with the story of a transgenic mouse in London called Randy. Why was this mouse so special? The mouse in question was a chromosomal female (XX) made male by the presence of a transgene containing the Y chromosome gene Sry. This sex-reversal provided clear experimental proof that Sry was the elusive mammalian sex-determining gene. Twenty-five years on, Koopman, Sinclair and Lovell-Badge reflect on what this discovery meant for our understanding of how males and females arise and what remains to be understood. Read the Spotlight on p. 1633
An overview of mammalian pluripotency
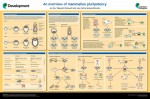 Mammalian pluripotency is the ability to give rise to all somatic cells as well as the germ cells of an adult mammal. Here, Juan Carlos Izpisua Belmonte and colleagues provide a summary of mammalian pluripotency both in vivoand in vitro, and highlight recent and future applications of pluripotent stem cells for basic and translational research. Read the poster article on p. 1644
Mammalian pluripotency is the ability to give rise to all somatic cells as well as the germ cells of an adult mammal. Here, Juan Carlos Izpisua Belmonte and colleagues provide a summary of mammalian pluripotency both in vivoand in vitro, and highlight recent and future applications of pluripotent stem cells for basic and translational research. Read the poster article on p. 1644
Neurogenesis in Cancun: where science meets the sea
 In March 2016, meeting organizers Sebastian Jessberger and Hongjun Song brought together over 100 scientists from around the world to Cancun, Mexico to present the latest research on neurogenesis. Here,
In March 2016, meeting organizers Sebastian Jessberger and Hongjun Song brought together over 100 scientists from around the world to Cancun, Mexico to present the latest research on neurogenesis. Here,


 (No Ratings Yet)
(No Ratings Yet)