Synthetic Human Embryology: The Rise of A New Era with New Collaborations
Posted by yueshao, on 9 January 2018
— A look behind the paper “A pluripotent stem cell-based model for post-implantation human amniotic sac development“
Early stages of human embryo development are vital for successful pregnancy and the health of the embryo. Abnormal early development often causes infertility as well as various birth defects. Despite its scientific and clinical importance, early development of the human embryo is poorly understood due to a lack of access to human embryo specimens in vivo. In addition, the drastic differences between human and other common animal models, e.g., mice, in certain developmental stages, such as implantation, has further limited advances in early human embryology. Given the limited accessibility, as well as ethical controversies surrounding research on intact human embryos, it is of paramount importance to seek in vitro synthetic methods for studying early human embryogenic events without using a biologically complete human embryo.
To achieve this goal, our labs, as well as other research laboratories, have endeavored to develop stem cell-based synthetic approaches for advancing the fundamental understanding of early human embryology. In this post, we will take you on a retrospective tour behind our recent work on a bioengineered in vitro model for post-implantation human amniotic sac development1, which we believe is a vivid example of the type of successful, multidisciplinary collaborative research that is critical for the rising field of Synthetic Human Embryology.
- Start from a void
This collaborative work started from an unexpected observation made by Ken Taniguchi in 2014, which revealed a potent capability of human pluripotent stem cells (hPSC) to form a lumen – an apical cavity surrounded by multiple polarized cells (hPSC-cyst) – in a culture dish under both 2D and 3D conditions2. This hPSC-cyst morphogenesis echoes the formation of an epiblastic cavity in early mammalian embryo3-5 and substantiates the potential of hPSC to model not only the differentiation but also the morphogenesis involved in early embryonic development. Inspired by this potential, we teamed up to explore something new: to reconstruct a human neural tube in a culture dish by generating 3D lumenal hPSC cysts/tubes and instructing them to undergo neuronal differentiation. However, our quest for a synthetic human neural tube turned out to be more elusive than we envisioned.
- Biomimetic embryoid model by hPSC self-organization in a soft 3D niche
(Yue Shao)
After a few months, despite a number of different chemical induction protocols that we tested, our efforts to make a synthetic human neural tube still fell short; we were unable to induce dorsoventral patterning, resulting in an elongated tube with a uniform cell fate throughout the structure. Inspired by our previous successes in modulating neural differentiation of hPSC in a 2D culture system by changing the mechanics of the culture substrate6, I, being a little desperate back then, did an experiment to test whether engineering the mechanical softness of our 3D neural cyst culture system might produce the desired results. To our disappointment, neural cysts did not exhibit any intrinsic dorsoventral patterning, regardless of the mechanics of the 3D culture environment.
Fortunately, that experiment was not a total failure. Something that happened in the control group (3D culture environment without neural induction) caught our eye. Surprisingly, when cultured on a soft basement membrane gel bed with a 3D matrix overlay, hPSC started to self-organize into cystic structures enclosed by a squamous epithelium, through a continuous tissue thinning process (Movie 1)1. Along with these squamous cysts, we also observed the formation of a small population of “asymmetric cysts”, which feature a thin, squamous epithelium at one side and a thick, columnar epithelium at the other side; in this case, cysts developed through an autonomous symmetry-breaking process (Movie 2)1. Both the squamous and asymmetric cysts, to the best of our knowledge, were new structures generated for the first time in a culture dish at the time. They lit up our excitement and our wonder: What are these structures? Do they resemble any early embryonic structures?
Many guesses were put on the table during our team discussion, but one image seemed to be the key to settle the guessing game. An image found in the Carnegie Collection of Human Embryos (through the Virtual Human Embryo database) clearly showed an asymmetric cystic structure lying at the center of a post-implantation (day 12) human embryo (Figure 1), with a squamous epithelium – the amniotic ectoderm – at its roof side, and a columnar epithelium – the pluripotent epiblast, or embryonic disc – at the floor side, enveloping the amniotic cavity within. This asymmetric amniotic sac will eventually develop into the human embryo, with the enveloping amniotic membrane, and therefore is a key structure for early human embryogenesis. Given the striking morphological similarity between the post-implantation amniotic sac and the asymmetric cyst seen in our engineered soft 3D niche, we could not help but wonder: did we just generate a synthetic human amniotic sac?
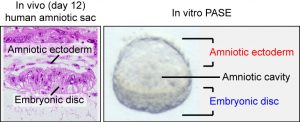
Figure 1: (left) Carnegie stage 5c (day 12) human embryo section, showing the amniotic ectoderm and the embryonic disc (pluripotent epiblast). Obtained from the Virtual Human Embryo project database. (right) Phase contrast image showing a representative asymmetric cyst with a distinct bipolar morphological pattern that mirrors the in vivo human amniotic sac.
Due to the lack of an in vivo dataset of human embryos at the implantation stage, we had to carefully wade through a stream of questions to conclude that “We really synthesized an amniotic sac structure”. With effort and expertise from a group of engineers, biologists, and bioinformaticians, we first demonstrated, to the extent that we could, the molecular similarity between the hPSC-derived squamous epithelium in vitro and first-trimester human amniotic ectoderm in vivo7. This finding was further confirmed by comparing our asymmetric cysts to a new dataset from post-implantation non-human primate embryo8. These results demonstrated that the asymmetric cyst – which we named the post-implantation amniotic sac embryoid, or PASE, shows significant molecular (e.g., asymmetric activation of BMP signaling) and morphological (e.g., bipolar segregation of amniotic ectoderm and epiblast markers) resemblance to the primate post-implantation amniotic sac. Despite these exciting findings, we are still in need of more early amniotic sac datasets to fully understand this embryoid and the extent of its biomimicry.
Although this journey already took us two and a half years, it only marks the beginning of a bigger scientific endeavor to understand early human embryonic development using synthetic approaches9. Without the need of live, intact human embryo specimen, our synthetic PASE platform can be leveraged to answer a myriad of scientific questions about an early human developmental stage that has been previously inaccessible.
- A model for deciphering the molecular and cellular blueprints of early human embryonic development
(Kenichiro Taniguchi)
Prior to this collaboration with Yue Shao and the laboratory of Dr. Jianping Fu, my research interest (working as a postdoctoral fellow in the laboratory of Dr. Deborah Gumucio) had been primarily in how cell polarity machinery regulates the formation or lumens during development. Initially my focus was on lumen formation in endodermally determined gut-like organoids, developed from hPSC. But I was struck by the fact that under a variety of conditions, my control cells (undifferentiated hPSC) also robustly formed lumens and grew into cystic structures! I speculated that, since hPSC resemble epiblast cells, perhaps this strong lumen-forming tendency represents the ability of the cells to self-organize to form an epiblast cavity. This was exciting, because the cell polarity aspect of human epiblast cavity formation had been relatively understudied, and for the first time, the hPSC-cyst model allowed us to pinpoint molecular and cellular processes associated with polarization of the early human epiblast, including involvement of actin cytoskeleton, as well as the highly unexpected role of an apicosome – a novel apically polarized organelle10.
While these studies led to exciting and fundamental discoveries in cell polarization, they also raised another interesting question: if this is the epiblast (or pro-amniotic) cavity, where is the amnion? Meanwhile, Yue and I were busily trying to figure out a protocol to generate dorsoventrally patterned neural tubes (which, as you know by now, was not quite successful). For my doctoral thesis work, I had worked on a mouse model of holoprocensephaly (a congenital forebrain malformation frequently caused by aberrant Shh signaling), so this was an enjoyable and stimulating project to work on. Using engineered substrates, we were getting beautiful long and branched continuous tubes of neural cells (we just could not get them patterned!). However, as Yue mentioned earlier, our non-neurally induced hPSC controls turned out to hold the most interesting data: when plated in a specific type of environment (on a thick soft gel bed, with surrounding extracellular matrix), we observed lumenal cysts composed of squamous cells. Since we were already “primed” to think about the lumen in pluripotent cysts as a potential pro-amniotic cavity, it wasn’t much of a stretch to imagine that these squamous cells just might be amnion.
As further biological analyses led us to confirm that these squamous cyst cells were indeed of amniotic lineage, I came to appreciate the power of engineering (and this highly productive collaboration) in developing these innovative controlled 3D culture conditions, and also in generating engineered substrates, such as PDMS microposts ,to decipher mechanical properties of amniotic differentiation. These engineering tools were instrumental in testing hypotheses that would have been difficult for pure biologists to envision. In fact, in this case, our further studies showed that the amniotic fate cascade that we observed is actually initiated by a purely mechanical trigger. The discovery of self-organizing amniotic organoids (hPSC-amnion and PASE) has now expanded our toolbox to explore early human embryogenesis.
Countless additional biological questions blossomed through the generation of PASE, including those concerning the mechanisms regulating a surprising gastrulation-like cell dissemination phenotype observed in mature PASE. During human embryonic development, a major step following the establishment of the asymmetric amniotic sac is gastrulation; indeed, we saw that many PASE exhibit cells disseminating from the columnar pluripotent embryonic disc-like domain (Movie 3)1. However, we did not know whether this cell dissemination was dependent on epithelial-to-mesenchymal transition (just like cells undergoing gastrulation). It is an exciting time in biology right now because we can relatively easily generate mutations in established cell lines using the CRISPR/Cas9 genome editing system11. Using a highly efficient CRISPR/Cas9 technique based on a piggyBac transposon system that we developed, we generated multiple hESC clones carrying different loss-of-function (LOF) mutations of the SNAI1 gene (a major transcriptional driver of EMT during gastrulation), and showed that the cell dissemination phenotype is significantly reduced in the SNAI1 LOF background. This finding confirms that, similar to gastrulation in vivo, EMT is a critical driver of cell dissemination in PASE. We are now actively seeking transcriptional regulators of associated processes, such as amnion fate determination, asymmetric morphogenesis and pluripotency maintenance.
These stages of peri- and early post-implantation human embryogenesis are critical for the continuation of a successful human pregnancy. The discovery that at least some of these early steps can be recapitulated in hPSC-derived embryoids/organoids provides an exciting new in vitro platform for advancing our understanding of early human embryogenesis. Clearly, however, future in vivo work will be required to validate findings in this model; perhaps some of this can be accomplished using techniques recently developed in Cynomolgus monkeys8. Scientifically speaking, we know that we have only touched the tip of the PASE biology iceberg and we have an endless list of questions that need to be answered.
- Concluding remarks
Looking back, this collaborative work was made possible only with the tremendous efforts from our scientific collaborators and consultants as well as all members of the Fu and Gumucio laboratories at the University of Michigan. As synthetic human embryology is bound to be a highly interdisciplinary course of study, we hope our case will not only serve as a stepping stone to advance this type of research, but also an encouragement for fellow engineers, developmental and cell biologists, mathematicians, physicists, physicians, etc., to join forces and initiate fruitful interactions and collaborations to advance discovery.
(Yue Shao, Kenichiro Taniguchi, Deborah Gumucio, Jianping Fu)
Movie Captions:
Movie 1: Time-lapse movie showing the development of a squamous cyst from hPSC, through a continuous tissue-thinning process that converts an initially columnar cyst into a fully squamous one. Time stamps indicate the total hours of culture. Scale bar, 50 µm. Adapted from the original publication by Nature Publishing Group1.
Movie 2: Time-lapse movie showing dynamic morphogenesis during the development of an asymmetric cyst (which we later identified and named as the post-implantation amniotic sac embryoid, or PASE). Time stamps indicate the total hours of culture. Scale bar, 50 µm. Adapted from the original publication by Nature Publishing Group1.
Movie 3: Time-lapse movie showing the progressive emergence of epithelial-to-mesenchymal transition and primitive streak-like phenotype in a PASE. Time stamps indicate the total hours of culture. Scale bar, 50 µm. Adapted from the original publication by Nature Publishing Group1.
References:
- Shao, Y., Taniguchi, K., Townshend, R. F., Miki, T., Gumucio, D. L. and Fu, J. A pluripotent stem cell-based model for post-implantation human amniotic sac development. Nat. Commun. 8, 208 (2017).
- Taniguchi, K., Shao, Y., Townshend, R. F., Tsai, Y. H., DeLong, C. J., Lopez, S. A., Gayen, S., Freddo, A. M., Chue, D. J., Thomas, D. J., Spence, J. R., Margolis, B., Kalantry, S., Fu, J. P., O’Shea, K. S. and Gumucio, D. L. Lumen formation is an intrinsic property of isolated human pluripotent stem cells. Stem Cell Rep. 5, 954-962 (2015).
- Bedzhov, I. and Zernicka-Goetz, M. Self-organizing properties of mouse pluripotent cells initiate morphogenesis upon implantation. Cell 156, 1032-1044 (2014).
- Shahbazi, M. N., Jedrusik, A., Vuoristo, S., Recher, G., Hupalowska, A., Bolton, V., Fogarty, N. M., Campbell, A., Devito, L. G., Ilic, D., Khalaf, Y., Niakan, K. K., Fishel, S. and Zernicka-Goetz, M. Self-organization of the human embryo in the absence of maternal tissues. Nat. Cell Biol. 18, 700-708 (2016).
- Shahbazi, M. N., Scialdone, A., Skorupska, N., Weberling, A., Recher, G., Zhu, M., Jedrusik, A., Devito, L. G., Noli, L., Macaulay, I. C., Buecker, C., Khalaf, Y., Ilic, D., Voet, T., Marioni, J. C. and Zernicka-Goetz, M. Pluripotent state transitions coordinate morphogenesis in mouse and human embryos. Nature 552, 239-243 (2017).
- Sun, Y. B., Aw Yong, K. M., Villa-Diaz, L. G., Zhang, X. L., Chen, W. Q., Philson, R., Weng, S. N., Xu, H. X., Krebsbach, P. H. and Fu, J. P. Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells. Nature Mater. 13, 599-604 (2014).
- Shao, Y., Taniguchi, K., Gurdziel, K., Townshend, R. F., Xue, X., Yong, K. M. A., Sang, J., Spence, J. R., Gumucio, D. L. and Fu, J. Self-organized amniogenesis by human pluripotent stem cells in a biomimetic implantation-like niche. Nature Mater. 16, 419-425 (2017).
- Sasaki, K., Nakamura, T., Okamoto, I., Yabuta, Y., Iwatani, C., Tsuchiya, H., Seita, Y., Nakamura, S., Shiraki, N., Takakuwa, T., Yamamoto, T. and Saitou, M. The germ cell fate of cynomolgus monkeys is specified in the nascent amnion. Dev. Cell 39, 169-185 (2016).
- Harrison, S. E., Sozen, B., Christodoulou, N., Kyprianou, C. and Zernicka-Goetz, M. Assembly of embryonic and extraembryonic stem cells to mimic embryogenesis in vitro. Science 10.1126/science.aal1810 (2017).
- Taniguchi, K., Shao, Y., Townshend, R. F., Cortez, C. L., Harris, C. E., Meshinchi, S., Kalantry, S., Fu, J., O’Shea, K. S. and Gumucio, D. L. An apicosome initiates self-organizing morphogenesis of human pluripotent stem cells. J. Cell Biol. 216, 3981-3990 (2017).
- Hsu, P. D., Lander, E. S. and Zhang, F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262-1278 (2014).


 (1 votes)
(1 votes)
prof premraj pushpakaran writes — 2018 marks the 100th birth year of Edward Butts Lewis!!!