Earning your Green stripes: enhancing sustainability in zebrafish research
Posted by Anya Suppermpool, on 6 January 2024
Within our collective of zebrafish labs at UCL, the sheer volume of single-use plastic Petri dishes we breeze through is staggering – a staggering 130 kg per year, to be exact. To put this into perspective, it’s a whopping 8.4% more plastic waste than the average person in the UK generates annually, and that’s solely from our Petri dish used for housing larvae. And petri dishes are not the only problem, we use thousands of plastic tips, tubes, PCR plates…and on and on. It’s a dilemma that resonates deeply with many of us in the scientific community. As sustainability takes centre stage across institutions, our host, UCL, has set commendable goals: aiming to eliminate non-essential single-use plastic on campus by 2024 and achieve net-zero carbon status by 2030. While sweeping institutional initiatives mark significant progress, the reality remains that not all these strides trickle down to our individual laboratories. In the scientific community, single-use plastic consumables are gold-standard, and ultracold storage is indispensable. Finding ourselves in this situation, all the fish groups sharing the communal labs got together and sat down to brainstorm ways to make our work more sustainable. We came up with ideas to reduce our environmental impact while continuing our research, in three main areas:
Plastics
Even though single-use plastic tips and Petri dishes aren’t yet on UCL’s sustainability radar for 2024, we’re already taking steps to reduce our use across the groups. Where possible, we are swapping plastic for glassware and when we can’t use glassware, we are finding ways to reuse plastic tips and petri dishes whenever we can. For instance, to reuse Petri-dishes for housing zebrafish embryos we remove labels with ethanol, soak them in hot water for at least 20’ and then dry, ready to be re-used! We’ve also tracked our plastic usage by weighing our Petri dish bins weekly. This not only gauges our sustainability efforts but also aids in estimating equipment stock levels.

One lab protocol that significantly contributes to plastic usage is genotyping, a routine procedure consuming lots of plastic tips and PCR plates. Not commonly known is the practice of reusing tips for gel loading, a neat plastic-saving tip resurrected by the more mature lab members involved in large genetic screens and manual mapping of mutants. These mapping projects required running copious amounts of PCRs on lots of embryos and we did it reusing the same tips again and again. Credit originally to William Talbot’s lab in Stanford University (the master mapper!) for this thrifty and sustainable tip!
Not quite sure how to re-use pipette tips? Check out our video at http://zebrafishucl.org/plastic for simple tips. Reusing tips can serve various purposes, from simple DNA digestion controls to PCR products and RNA checks – any application where gel band extraction or subsequent analysis isn’t necessary. To reduce plastic use further, we also keep partially-used PCR plates for future runs. Start now by collecting used tips in a dedicated box for hassle-free diagnostic gel loading today!
And it’s not only for loading gels, pipette tips can have multiple lives. From water and buffers to alcohol and various solutions that are often used, tips can be reused! Everyone has their unique and fun way to store pipette and Pastette tips – discover yours or explore some ideas here.
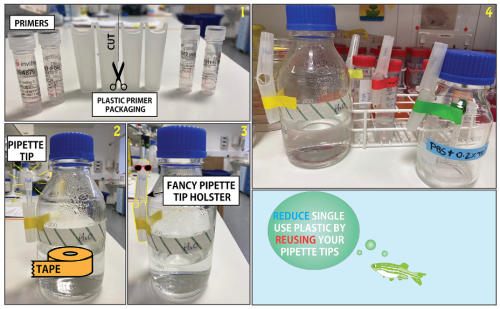
Now that we can re-use some tips, what about plastic tubes and falcons? We all use lots of Eppendorf tubes and there isn’t much we can do about this. At a recent sustainability event at the Royal Society, Eppendorf’s life cycle assessment delivered a sobering result – their biobased tubes only show an underwhelming reduction of 16% in CO2 compared to their standard fossil-based counterpart. While there’s a projected 27% decrease in this, the current findings are disappointing. Not to mention the biobased tips are unfortunately more expensive.
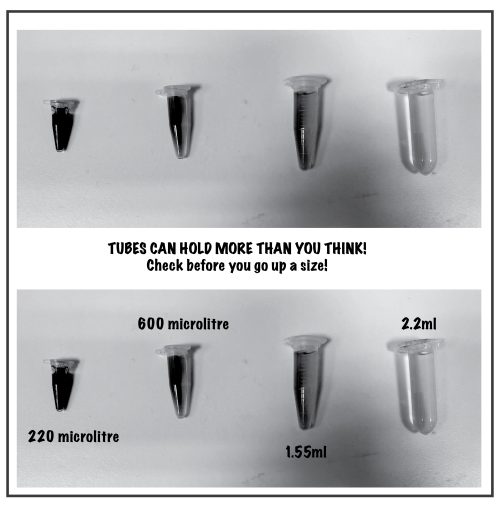
As we left the conference, we felt there was room for improvement. How often do we use a classic 1.5 mL Eppendorf for a miniscule 50 µL sample or for storing small aliquots of enzymes and antibodies? Turns out, the comfortably-stored volume for:
- PCR tube – 0.220 mL
- 0.5 mL Eppendorf – 0.6 mL
- 1.5 mL Eppendorf – 1.6 mL
- 2 mL Eppendorf – 2.2 mL
Therefore, by just considering the right tube size for an experiment, instead of defaulting to the larger more commonly used 1.5 mL could drastically reduce our plastic usage and associated CO2 footprint. It seems we have the power to make a bigger impact than even Eppendorf can achieve currently, with minimum effort.
The principles of “reduce and reuse” can also be applied to other areas of the lab such as reagents. For diagnostics requiring only a few lanes, consider using a smaller gel. Here, we laser-cut a miniature electrophoresis gel mould for running extra small gels by repurposing our old/broken electrophoresis chamber plastics lids that uses only a third of agarose compare to our smallest commercially bought mould. You can download the laser cutting template from our website here.
Energy
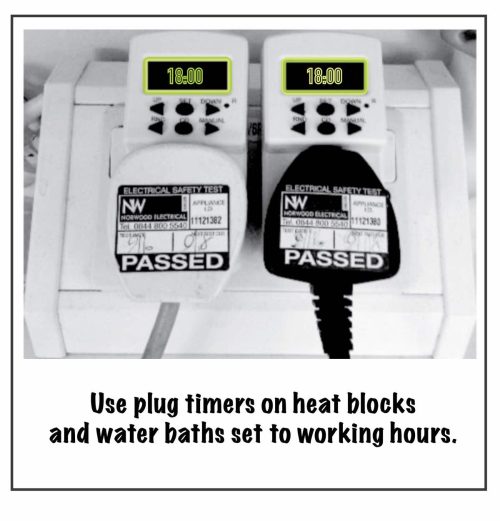
We reduce energy consumption by turning off equipment when not in use, utilizing energy-efficient appliances, and optimizing equipment settings. This can be as simple as switching freezers from -80°C to -70°C (and reduce 30-40% energy consumption!) or switching off PC monitors after work, to deleting any useless data accumulating in the cloud! We also find that installing plug timers set to working hours on appliances such as water baths and heat blocks works really well.
Certain labs are still hesitant to switch ultra-low temperature freezer to -70°C because of sample safety concerns. We store our plasmid and antibodies stocks as well as most of our RNA at -20°C while total RNA, tissues, and competent cells are stored at -70°C. And we’re not the only ones, check out this list of samples successfully stored at higher temperatures by universities in the US. Increasing number of labs have now made the switch. Start 2024 right by joining a sustainability framework like the Freezer Challenge.
It’s always useful to regularly rethink ingrained lab practice. Some procedures appear to be engrained without clear origins. Take, for instance, the ‘cold hold’ – the infinite hold step at the end of a PCR protocol at the 4˚ C. Considering that our PCR products are double-stranded DNA, a fairly stable molecule, the need for this step becomes questionable. Don’t believe us? Here’s the evidence. Now, we’ve opted to terminate the cycle after the final extension, not only conserving energy but also prolonging the machine’s lifespan!
Culture of change
To maintain a consistent and concerted effort toward sustainability, we initiated a floor-wide green committee comprising volunteers from each of our labs. This committee also serves as a bridge between labs and core facilities, such as the UCL Fish Facility. Expanding on this initiative, we’ve established similar committees at the Departmental level, ensuring sustainability remains a priority. As part of this commitment, every new member, including students, postdocs, and PIs, undergoes sustainability training, and we’ve mandated plastic-free events throughout the department (take your own glass to socials, for instance). Our efforts are bolstered by frameworks like the laboratory efficient assessment framework (LEAF) which standardises sustainable practices. We joined LEAF in 2018 and thanks to contributions from many participants, the framework has evolved over the years and will keep improving and expanding. Being part of a framework that is endorsed by leading scientists and that is recognised by funding bodies helps with the acquisition, reinforcement and spreading of good, ideally, GOLD practices. Our entire department has set its sights on having all laboratories taking part of the LEAF and achieving Gold awards by 2025, a testament to our commitment to sustainability.
Despite having come a long way, there is still lots to do including in the way we think about how we conduct and publish research. Open research practices and open access publishing is indeed helping scientists to have better access to data, methods and resources and to minimise unnecessary duplication (while acknowledging that validating results is of course critical!). Sharing protocols and reagents pre-publication as well as publishing negative results should become the norm and we would love to see sustainability approaches within the methods section of papers (including positive as well as the negative outcomes).
While lots of science will remain competitive and sometimes secretive, the balance is shifting to more cooperative, collaborative, sustainable research with funders recognising the importance of community projects and resources that bring widespread benefits to the global research endeavour.
Useful links and resources
Re-using pipette tips for diagnostic gel loading
LEAF – UK-based labs
The Freezer Challenge – North American labs
Co-written by: Gaia Gestri and Anya Suppermpool (UCL)
Images: Kate Turner (UCL)


 (8 votes)
(8 votes)