In Development this week (Vol. 139, Issue 7)
Posted by Seema Grewal, on 6 March 2012
Here are the research highlights from the current issue of Development:
miR-125 seals hESC neural fate
MicroRNAs, small non-coding RNAs, have recently emerged as key regulators of embryonic development. In particular, they can coordinate cell fate determination by blocking alternative cell fate choices. Here (p. 1247), Alexandra Benchoua and colleagues report that the microRNA miR-125 contributes to the neural specification of pluripotent human embryonic stem cells (hESCs). By using a culture system that promotes hESC neuralization, the researchers show that miR-125 is expressed in a time window compatible with a role in neural commitment in vitro. They show that miR-125 promotes the conversion of pluripotent cells into SOX1-positive neural precursors by, at least in part, blocking the expression of SMAD4, a key regulator of pluripotent stem cell lineage commitment that promotes non-neural cell fates. Finally, the researchers show that expression of miR-125 is responsive to the level of TGFβ-like molecules. Together, these results identify a central role for miR-125 in the irreversible neural lineage commitment of pluripotent stem cells in response to external stimuli.
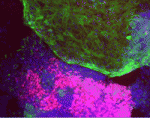
GLE1: keeping neural precursors alive
Lethal congenital contracture syndrome 1 (LCCS1) is a prenatally fatal, autosomal recessive human disorder. Affected foetuses have multiple defects, including limb deformities (contractures), loss of voluntary muscle movement and a distinct neuropathology. Mutations in GLE1, which encodes a protein involved in mRNA export and translation, have been implicated in LCCS1 and, on p. 1316, Susan Wente and co-workers investigate the link between Gle1 function and LCCS1 pathology using zebrafish as a model system. They report that disruption of Gle1 function produces phenotypes in zebrafish embryos that parallel those of human LCCS1 foetuses, including a reduction of motoneurons and aberrant arborization of motor axons. Surprisingly, the researchers report, apoptosis of neural precursors, rather than degeneration of differentiated neurons, as previously suggested, causes the motoneuron deficiency. The researchers propose, therefore, that rapidly dividing cells, including organ precursors in both neuronal and non-neuronal tissue, have a high demand for Gle1 activity, and that apoptosis of these precursors because of Gle1 deficiency produces the pleiotropic abnormalities seen in LCCS1 foetuses.
Separating karrikin and strigolactone effects

Karrikins are smoke-derived butenolides that, by stimulating seed germination and enhancing seed responses to light, allow plants to exploit reduced competition for light, water and nutrients after wildfires. By contrast, strigolactones are plant-derived butenolides that regulate shoot and root architecture. In Arabidopsis, responses to both classes of molecule require the F-box protein MAX2, so how are the physiologically distinct responses to karrikins and strigolactones achieved? Here, Mark Waters and colleagues suggest that the answer to this puzzle lies with evolutionary specialization within the DWARF14 superfamily of α/β hydrolases (see p. 1285). In rice, strigolactone-dependent inhibition of shoot branching requires DWARF14. The researchers show that, in Arabidopsis, the DWARF14 orthologue AtD14 is necessary for normal strigolactone responses, whereas the AtD14 paralogue KAI2 is required for karrikin responses. Notably, the expression patterns of AtD14 and KAI2 are consistent with the plant’s capacity to respond to specific growth regulators at different developmental stages. Thus, AtD14 and KAI2 define proteins that permit the separate regulation of strigolactone and karrikin signalling by MAX2.
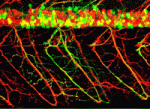
Foxj1: Not(o) enough for node function

During embryonic patterning in amniotes, cilia in the node generate a leftward flow of extra-embryonic fluid that establishes left-right asymmetry. Now, on p. 1276, Achim Gossler and co-workers report that Noto and Foxj1 (two key transcription factors that are expressed in the node) differentially regulate mouse node formation, nodal ciliogenesis and cilia positioning. Noto, which controls node morphogenesis, nodal ciliogenesis and left-right asymmetry in mice, acts upstream of Foxj1 but the role of Foxj1 in nodal ciliogenesis is unclear. To address this issue, the researchers analyse mouse embryos deficient for Foxj1 and embryos in which the Foxj1-coding sequence replaces the Noto-coding sequence. Foxj1 expressed from the Noto locus is functional and restores the formation of motile nodal cilia. However, in the absence of functional Noto, Foxj1 is insufficient for the correct positioning of these cilia and cannot restore normal node morphology. Thus, the researchers conclude, Noto regulates nodal ciliogenesis through Foxj1 but regulates node morphogenesis and cilia localization independently of Foxj1.
Engrailed 1 shapes the skull

Bones in the vertebrate skull are connected by cranial sutures: flexible fibrous joints that are essential for normal postnatal brain growth. The coronal suture separates the parietal and frontal bones, which are derived from cephalic paraxial mesoderm (Mes) cells and neural crest (NeuC) mesenchyme, respectively. But where do the mesenchymal precursors that generate the coronal suture come from? Ron Deckelbaum, Cynthia Loomis and colleagues (see p. 1346) use genetic fate mapping to show that, in mice, these precursors originate from hedgehog-responsive Mes cells that migrate into a supraorbital domain to establish a lineage boundary with NeuC mesenchyme. Importantly, the researchers show that the transcription factor Engrailed 1 (En1) regulates cell movement and NeuC/Mes lineage boundary positioning during coronal suture formation, and also prevents premature osteogenic conversion of the sutural mesenchyme by controlling the level of fibroblast growth factor receptor 2. Further investigation of these molecular mechanisms could lead to cell-based therapies for craniosynostosis (premature suture closure), which can cause craniofacial deformities and impaired brain development.
Well wrapped: vimentin regulates myelination
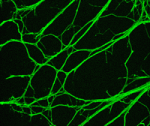
Myelination of peripheral nerves, which is essential for the rapid and efficient propagation of electrical messages along axons, has to be carefully controlled during development. However, the molecular mechanisms that determine myelin sheath thickness are only partly understood. Here (p. 1359), Stefano Carlo Previtali and colleagues report that the intermediate filament protein vimentin negatively regulates peripheral nerve myelination. Vimentin is highly expressed in Schwann cells and neurons during embryonic development and during nerve regeneration. The researchers show that loss of neuronal vimentin results in peripheral nerve hypermyelination in transgenic mice and in a myelinating co-culture system. This increased myelin sheath thickness occurs, they report, due to an increase in the levels of axonal neuregulin1 (NRG1) type III, a key regulator of myelin formation in peripheral nerves. Finally, they show that vimentin acts synergistically with the protease TACE to regulate NRG1 type III levels. Together, these results provide new insights into peripheral nerve myelination and identify potential targets for the treatment of peripheral neuropathies.
Plus…
Fluid flows and forces in development: functions, features and biophysical principles
Cells are subjected to various forces during morphogenesis, including those resulting from microscopic fluid flows. Here, Julien Vermot and colleagues review the biomechanical features and the physiological functions of biological fluid flows during development. See the Review article on p. 1229.



 (1 votes)
(1 votes)