A day in the life of a Trichoplax lab
Posted by Marvin Leria, on 14 December 2022
Hi, my name is Marvin Leria and I’m a PhD student, funded by Turing Center for Living Systems (CENTURI), at Aix-Marseille University in Marseille, France. I work in the lab of André Le Bivic under the supervision of Andrea Pasini, and co-supervised by Raphael Clément. Our lab is located in the magnificent Calanques National Park by the Mediterranean Sea, which offers a peaceful environment to do research (Figure 1). The main research topics of our lab revolve around cell polarity, morphogenesis and the evolution of epithelia. Historically, our lab has worked with cell cultures, but we are now developing new models such as marine sponges and more recently placozoans, to highlight conserved features and innovations in epithelial evolution.

Presentation of placozoans
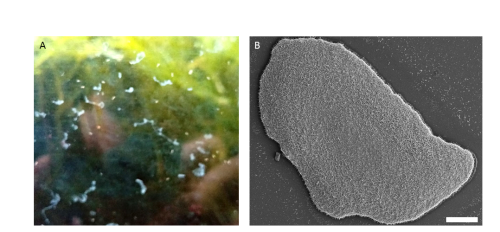
Placozoans are small and flat (around 1-2 mm in diameter, 20-30 µm thick) benthic marine animals (Figure 2.B) that are found around the world, mainly in tropical and subtropical areas such as coral reefs, mangroves, etc. (Schierwater et al., 2021). They are found gliding on rocks and other substrates and they mainly feed upon biofilms containing algae, bacteria and other microorganisms by means of external digestion (Figure 2.A)
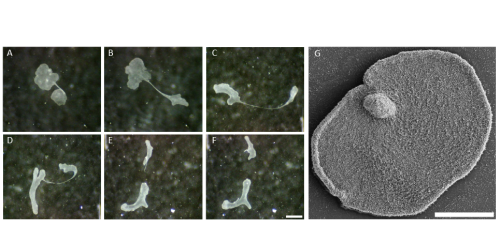
The life cycle of placozoans remains very enigmatic. Reproduction mainly occurs asexually, generally either by binary fission or by budding which produces juvenile animals referred to as swarmers (Figure 3; Thieman and Ruthman, 1991; Eitel et al., 2011; Zuccolotto-Arellano and Cuervo-González, 2020). Little is known about sexual reproduction and embryogenesis even though it is thought to occur in nature (Signorovitch et al., 2005; Eitel et al., 2011).
Phylogeny and evolution of Placozoa
The first discovered placozoan species Trichoplax adhaerens was described by the German zoologist Franz Eilhard Schulze in 1883 (Schulze, 1883). Very recently, three other placozoan species have been described, Hoilungia hongkongensis (Eitel et al., 2018), Polyplacotoma mediterranea (Osigus et al., 2019) and Cladtertia collaboinventa (Neumann et al., 2022). Other placozoan haplotypes have been genetically distinguished based on mitochondrial 16S rDNA fragments (Voigt et al., 2004; Signorovitch et al., 2006; Eitel et al., 2013 (review); Osigus et al., 2019; Miyazawa et al., 2021). The genome of Trichoplax adhaerens was sequenced and has been available since 2008 (Srivastava et al., 2008). It is one of the smallest animal genomes. It is composed of ~98 million base pairs and contains about 11,500 protein coding genes. In contrast, Trichoplax mitochondrial genome is one of the largest in the animal kingdom (Dellaporta et al., 2006). The phylogenetic position of placozoans among early-diverging phyla has been enormously controversial and still remains an important topic.
Body plan, cell morphology and physiology
Placozoans have no symmetry axis (only a top-bottom axis), and are devoid of muscles and nervous system. Their simple body plan consists of two monociliated epithelial layers, commonly referred to as the lower (dorsal) epithelium and the upper (ventral) one, with an internal cavity. Six morphologically distinct cell types have been described so far: upper epithelial cells, lower epithelial cells, gravity-sensing crystal cells, digestive enzyme-secreting lipophil cells, mucous-secreting and peptidergic gland cells and internal phagocytic fiber cells (Figure 4; Smith et al., 2014; Mayorova et al., 2019; Mayorova et al., 2021).
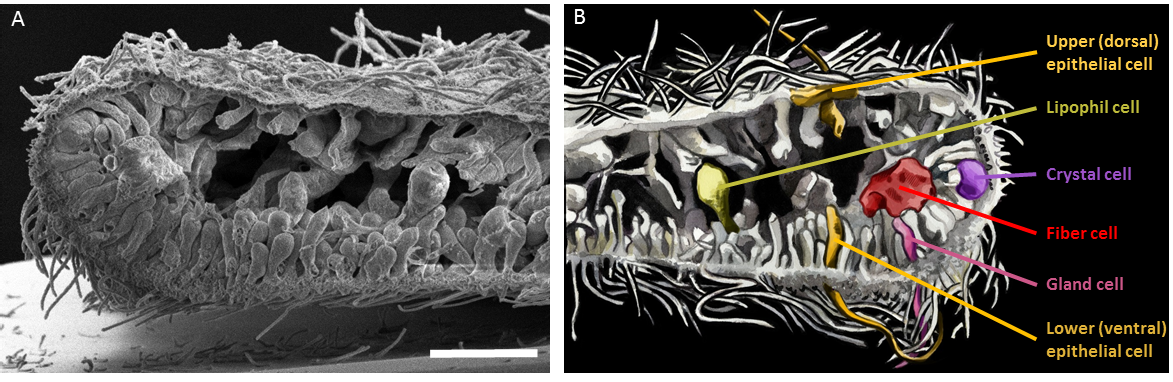
Surprisingly, despite the absence of a nervous system, some epithelial gland cells also synthesize neuropeptide-like molecules, which appear to control not only the behaviour of Trichoplax, but also its shape (Varoqueaux et al., 2018). Indeed, placozoans are tiny experts in shapeshifting and are able to adopt an incredible range of diverse shapes (see Figure 2 and Figure 3). How it is possible for placozoans to change their shape while maintaining the integrity of their epithelia is a mystery (see Video 1), and trying to understand this mechanism is the main goal of my thesis work. It is very likely that the shapeshifting ability of placozoans depends on the unusual features of their epithelia, which have no basal lamina and only one type of intercellular junctions similar to adherens junction (Smith and Reese, 2016). This is why most of my work focuses on studying the epithelial organization and will give a comprehensive insight into Trichoplax epithelial evolution and biology.
Video 1: The shape of Trichoplax is changing over time while the animal is moving (the movie is 10-time accelerated).
My day in the lab
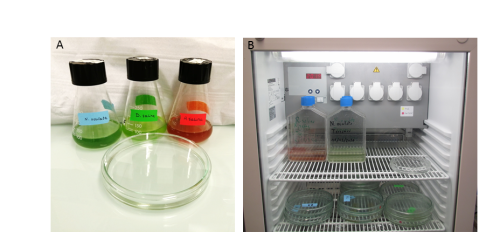
You may have read other contributors to ‘A day in the life of’ describing their adventurous trips to exotic places or wonderful seaside locations to collect their favourite organisms. Things are a bit less fancy for us, since we recovered our Trichoplax from the aquaria of a local tropical fish store in downtown Marseille. To collect animals for our first cultures, we deposited a few glass slides in a sea water tank and leave them there until a biofilm had developed. After several weeks, we took the slides back to the lab and observed some placozoans grazing on them! We now culture our placozoans in the lab in large petri dishes and feed them every week with their favourite red and green microalgae (Figure 5.A). They are kept at 20°C with a dark-light cycle (Figure 5.B). They need to be transferred regularly to new dishes when the old ones get dirty or when their density is getting high. We survey them every day to make sure that the culture living conditions are optimal.
For experiments, I transfer the Trichoplax carefully into a drop of sea water on coverslips and let them adhere properly. Once they are ready, I perform immunostaining experiments. Trichoplax are very fragile animals and experiments demand extreme patience and perseverance. After quite some efforts, I have succeeded in setting up fixation protocols that allow me to perform beautiful immunofluorescence staining of the epithelial cells and I can now follow how they change their shapes according to the changes of the whole animal. For this, I mostly use techniques such as confocal microscopy, image analysis and some electron microscopy too.
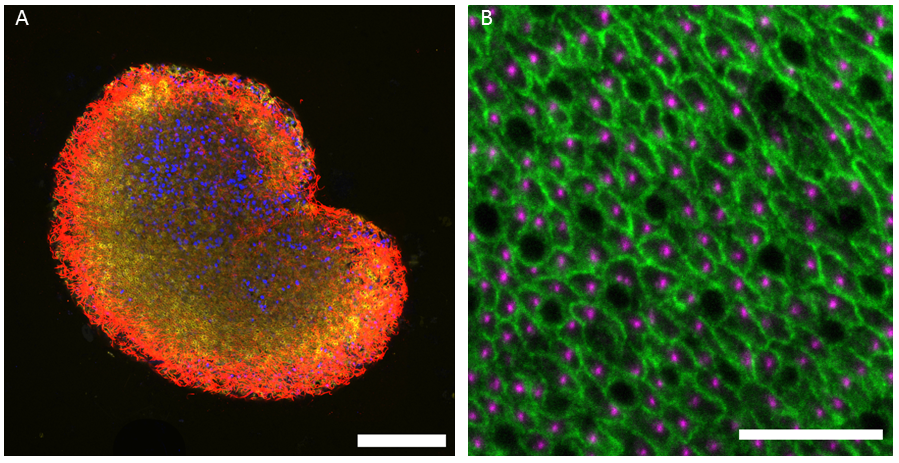
Challenges and perspectives
Establishing a new model organism is really challenging! Often, there are not many tools available and protocols have to be set up almost from scratch. It took me a while to start getting good results. But it is also very exciting! There are so many things that are yet to be discovered in placozoans which would help us to complete the puzzle of early animal evolution. I am really looking forward to exploring and finding interesting new things!
Our lab: https://www.ibdm.univ-amu.fr/team/evolution-and-morphogenesis-of-epithelia/#
References
Eitel M., Guidi L., Hadrys H., Balsamo M., & Schierwater B. (2011). New insights into placozoan sexual reproduction and development. PloS One, 6(5), e19639. https://doi.org/10.1371/journal.pone.0019639
Eitel M, Osigus HJ, DeSalle R, Schierwater B. Global diversity of the Placozoa. PLoS One. 2013;8(4):e57131. doi: 10.1371/journal.pone.0057131. Epub 2013 Apr 2. PMID: 23565136; PMCID: PMC3614897.
Eitel, M., Francis, W. R., Varoqueaux, F., Daraspe, J., Osigus, H. J., Krebs, S., Vargas, S., Blum, H., Williams, G. A., Schierwater, B., & Wörheide, G. (2018). Comparative genomics and the nature of placozoan species. PLoS biology, 16(7), e2005359. https://doi.org/10.1371/journal.pbio.2005359
Dellaporta, S. L., Xu, A., Sagasser, S., Jakob, W., Moreno, M. A., Buss, L. W., & Schierwater, B. (2006). Mitochondrial genome of Trichoplax adhaerens supports placozoa as the basal lower metazoan phylum. Proceedings of the National Academy of Sciences of the United States of America, 103(23), 8751–8756. https://doi.org/10.1073/pnas.0602076103
Mayorova, T. D., Hammar, K., Winters, C. A., Reese, T. S., & Smith, C. L. (2019). The ventral epithelium of Trichoplax adhaerens deploys in distinct patterns cells that secrete digestive enzymes, mucus or diverse neuropeptides. Biology open, 8(8), bio045674. https://doi.org/10.1242/bio.045674
Mayorova, T. D., Hammar, K., Jung, J. H., Aronova, M. A., Zhang, G., Winters, C. A., Reese, T. S., & Smith, C. L. (2021). Placozoan fiber cells: mediators of innate immunity and participants in wound healing. Scientific reports, 11(1), 23343. https://doi.org/10.1038/s41598-021-02735-9
Miyazawa, H., Osigus, H. J., Rolfes, S., Kamm, K., Schierwater, B., & Nakano, H. (2021). Mitochondrial Genome Evolution of Placozoans: Gene Rearrangements and Repeat Expansions. Genome biology and evolution, 13(1), evaa213. https://doi.org/10.1093/gbe/evaa213
Neumann, J.S., Tessler, M., Kamm, K., Osigus, H.J., Eshel, G., Narechania, A., Burns, J., DeSalle, R. & Schierwater, B. (2022). Phylogenomics and the first higher taxonomy of Placozoa, an ancient and enigmatic animal phylum. Frontiers in ecology and evolution. doi: 10.3389/fevo.2022.1016357
Osigus, H. J., Rolfes, S., Herzog, R., Kamm, K., & Schierwater, B. (2019). Polyplacotoma mediterranea is a new ramified placozoan species. Current biology: CB, 29(5), R148–R149. https://doi.org/10.1016/j.cub.2019.01.068
Schierwater, B., Osigus, H. J., Bergmann, T., Blackstone, N. W., Hadrys, H., Hauslage, J., Humbert, P. O., Kamm, K., Kvansakul, M., Wysocki, K., & DeSalle, R. (2021). The enigmatic Placozoa part 2: Exploring evolutionary controversies and promising questions on earth and in space. BioEssays : news and reviews in molecular, cellular and developmental biology, 43(10), e2100083. https://doi.org/10.1002/bies.202100083
Schulze, F.E. (1883). Trichoplax adhaerens, nov. gen., nov. spec. Zoologischen Anzeiger. 6, 92-97.
Signorovitch, A. Y., Dellaporta, S. L., & Buss, L. W. (2005). Molecular signatures for sex in the Placozoa. Proceedings of the National Academy of Sciences of the United States of America, 102(43), 15518–15522. https://doi.org/10.1073/pnas.0504031102
Signorovitch, A. Y., Dellaporta, S. L., & Buss, L. W. (2006). Caribbean placozoan phylogeography. The Biological bulletin, 211(2), 149–156. https://doi.org/10.2307/4134589
Smith, C. L., Varoqueaux, F., Kittelmann, M., Azzam, R. N., Cooper, B., Winters, C. A., Eitel, M., Fasshauer, D., & Reese, T. S. (2014). Novel cell types, neurosecretory cells, and body plan of the early-diverging metazoan Trichoplax adhaerens. Current biology : CB, 24(14), 1565–1572. https://doi.org/10.1016/j.cub.2014.05.046
Smith, C. L., & Reese, T. S. (2016). Adherens Junctions Modulate Diffusion between Epithelial Cells in Trichoplax adhaerens. The Biological bulletin, 231(3), 216–224. https://doi.org/10.1086/691069
Srivastava, M., Begovic, E., Chapman, J. et al. (2008) The Trichoplax genome and the nature of placozoans. Nature 454, 955–960 (2008). https://doi.org/10.1038/nature07191
Thiemann, M., Ruthmann, A. Alternative modes of asexual reproduction in Trichoplax adhaerens (Placozoa). Zoomorphology 110, 165–174 (1991). https://doi.org/10.1007/BF01632872
Varoqueaux, F., Williams, E. A., Grandemange, S., Truscello, L., Kamm, K., Schierwater, B., Jékely, G., & Fasshauer, D. (2018). High Cell Diversity and Complex Peptidergic Signaling Underlie Placozoan Behavior. Current biology : CB, 28(21), 3495–3501.e2. https://doi.org/10.1016/j.cub.2018.08.067
Voigt, O., Collins, A. G., Pearse, V. B., Pearse, J. S., Ender, A., Hadrys, H., & Schierwater, B. (2004). Placozoa — no longer a phylum of one. Current biology: CB, 14(22), R944–R945. https://doi.org/10.1016/j.cub.2004.10.036
Zuccolotto-Arellano, J., & Cuervo-González, R. (2020). Binary fission in Trichoplax is orthogonal to the subsequent division plane. Mechanisms of development, 162, 103608. https://doi.org/10.1016/j.mod.2020.103608


 (10 votes)
(10 votes)