A regeneration retrospective: muscle memory lane
Posted by Alex Eve, on 4 September 2023
This post is part of the regeneration retrospective series.
Unlike many other mammalian tissues, adult skeletal muscle has a remarkable aptitude for regeneration. Even after severe and repeated damage, functional skeletal muscle can be regenerated within a month. Such robust skeletal muscle restoration has been attributed to a population of Pax7+ stem/progenitor cells named satellite cells, which proliferate in response to injury, differentiate and fuse to generate mature muscle fibres – the mature, multinucleated, functional cells of the muscle (Collins and Kardon, 2021). In this second instalment, I discuss the highlights from the following two articles:
Cell populations in skeletal muscle after regeneration
J. C. T. Church
https://doi.org/10.1242/dev.23.2.531
J. C. T. Church spent most – if not all – of his career in East Africa, with some time at Makerere University College Medical School, Uganda, and then at University College in Nairobi, Kenya, where he worked as an orthopaedic surgeon. When not in the hospital, the “enthusiastic teacher” saw it fit to poke holes in the local population of fruit bats (Church and Noronha, 1965). In 1970, JEEM/Development published J.’s latest revelations from the fruit bat Eidolon helvum regarding the ability of skeletal muscle to regenerate following injury. Back then, satellite cells were well known but it remained to be determined whether they were indeed the source of restoring muscle fibre nuclei (myonuclei) during regeneration. Muscle regeneration had also largely been descriptive and, in his publication, Church provided perhaps the first quantitative report of cell populations in the skeletal muscle using electron-microscopy (EM; Fig. 1). Church’s approach was to perform single or repeated muscle ‘crush lesion’ experiments, followed by light and EM imaging, to identify and count the ratio of satellite cells to myonuclei. Although the number of muscle fibres remained stable, Church observed increased satellite cells, fibroblasts and myonuclei during regeneration following both single and double lesions. Overall, this study suggested that satellite cells could provide myonuclei to restore muscle fibres and, importantly, self-renew to sustain the population and regenerative response – a key behaviour of stem/progenitor cells.
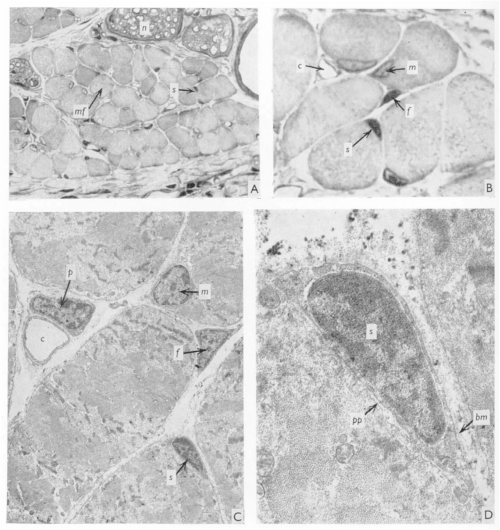
Let us now leap forward 50 years or so. Fortunately for chiropterophiles, fruit bats are now an uncommon model system for skeletal muscle regeneration research (and regenerative biology in general), with mice and zebrafish a more popular choice instead. It took the invention of inducible genetic labelling and ablation of satellite cells in the 2000s to finally confirm that satellite cells are necessary and sufficient for muscle regeneration (Collins and Kardon, 2021). EM has been complemented by state-of-the-art imaging of genetically manipulated (e.g. transgenic reporter) animals and it’s become increasingly evident that other cell populations, in addition to satellite cells, are needed for regeneration. Whether satellite cells contribute to muscle homeostasis, however, has been far less clear.
Hox11-expressing interstitial cells contribute to adult skeletal muscle at homeostasis
Corey G. K. Flynn, Paul R. Van Ginkel, Katharine A. Hubert, Qingyuan Guo, Steven M. Hrycaj, Aubrey E. McDermott, Angelo Madruga, Anna P. Miller and Deneen M. Wellik
https://doi.org/10.1242/dev.2901026
Flynn and colleagues’ study (University of Wisconsin School of Medicine and Public Health), published in Development earlier this year (Flynn et al., 2023), comes at a time of some conflicting evidence in the field when the ablation of satellite cells in adult muscle may or may not lead to muscle degeneration – even in the absence of injury. Evidence that muscle homeostasis is preserved when satellite cells are lost suggests that some other stem/progenitor population can contribute myonuclei for tissue maintenance. Using immunofluorescence and genetic labelling, Flynn and colleagues show that a population of Hoxa11-expressing interstitial stromal cells directly contribute to muscle fibres postnatally. Through genetic lineage-tracing experiments, the team confirm that this Hoxa11-expressing population doesn’t go through a satellite cell-like state (i.e. they are not Pax7+) and, therefore, they are an independent progenitor population. In response to injury, Hoxa11-lineage cells minimally contribute to muscle generation (Fig. 2), contributing to the growing body of evidence of satellite cell-independent muscle formation.
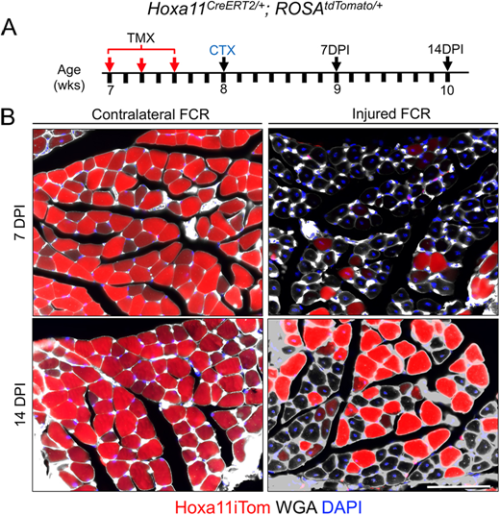
These articles illustrate a joint interest in understanding the cells that give rise to functional muscle. While J. C. T. Church contributed to the knowledge of satellite cells as a stem/progenitor population in regenerating muscle, the search for muscle-generating cell populations still continues many (many) years later, with Flynn and colleagues identifying a whole new population of Hoxa11-expressing cells that maintain muscle homeostasis. Join me again tomorrow for another trip down memory lane, when we look at early studies of wound healing and the important role of the extracellular matrix.
References
J. C. T. Church, R. F. Noronha. The use of the fruit bat in surgical research. East Afr Med J. July 1965; 42 (7): 348-55.
J. C. T. Church; Cell populations in skeletal muscle after regeneration. Development 1 April 1970; 23 (2): 531–537. doi: https://doi.org/10.1242/dev.23.2.531
Brittany C. Collins, Gabrielle Kardon; It takes all kinds: heterogeneity among satellite cells and fibro-adipogenic progenitors during skeletal muscle regeneration. Development 1 November 2021; 148 (21): dev199861. doi: https://doi.org/10.1242/dev.199861
Corey G. K. Flynn, Paul R. Van Ginkel, Katharine A. Hubert, Qingyuan Guo, Steven M. Hrycaj, Aubrey E. McDermott, Angelo Madruga, Anna P. Miller, Deneen M. Wellik; Hox11-expressing interstitial cells contribute to adult skeletal muscle at homeostasis. Development 15 February 2023; 150 (4): dev201026. doi: https://doi.org/10.1242/dev.201026


 (No Ratings Yet)
(No Ratings Yet)