An interview with Adam Shellard, winner of the 2022 BSCB Postdoctoral Research Medal
Posted by the Node, on 8 August 2022
Adam Shellard, a postdoc in Roberto Mayor’s lab, was the winner of the 2022 BSCB Postdoctoral Research Medal. We caught up with Adam over Teams to find out more about his career path so far, his evolving research interests and the Cell Migration webinar series that he started during the pandemic.

Where are you originally from?
I grew up in London, before going to the University of Manchester for my undergraduate studies. As part of this course, I completed a year-long internship at Thomas Jefferson University in Philadelphia, USA in Renato Iozzo’s lab. I spent a lot of time doing western blots and qPCRs but it was a great experience, both in the lab and having the opportunity to travel.
Why did you choose Roberto Mayor’s lab for your PhD?
I was on the Wellcome Trust Stem Cell and Developmental Biology programme at UCL, which meant I spent my first year doing rotations in different labs. When I started on the programme, I was interested in everything and didn’t have a special interest in any particular topic. I tried to choose labs where I could learn different techniques. I went to a lab that did more biochemistry; I went to one that used electron microscopy; and I did mouse work and live imaging for the first time. The rotations were a great opportunity to try lots of different techniques and topics to discover what I was most interested in. In the end, a big reason for choosing Roberto’s lab was that it was a good environment, and I really liked the people there. The topic didn’t matter so much at that stage because I felt that I could become interested in anything! I liked the fact they had lots of microscopes, and a lot of cool projects were going on at that time.
Can you tell us about your PhD research?
When I started my PhD, I was supervised by Elena Scarpa, who is now a group leader in Cambridge. She had some preliminary data showing an actomyosin cable around the edge of a neural crest cell cluster when it was dissected out and imaged in vitro. My project was to look at the role of actin and myosin during neural crest cell migration as we didn’t know anything about it. This sounds a little crazy, because obviously actin and myosin play a role in migration, but we didn’t know much about how they were involved in the collective migration of the neural crest. So, it started from there. I tried lots of experiments and whilst they worked, there were a lot of negative results in the first three years. Then when I got to the final year, luckily, or serendipitously, a couple of techniques that I’d been trying to work out for a long time, started to work. I finally got laser ablations working on the microscope after searching for so long, so I could very specifically test actomyosin cable function. At the same time, Xavier Trepat’s lab had published some optogenetic constructs which controlled contractility, so I cloned those and used them as well. What we found was that the neural crest, as a cluster, had an actomyosin cable around its edge. And in the absence of any chemoattractant, the cable would contract around the edge, so it would look like the cluster was pulsing. But if you put on a chemoattractant like SDF1 and the cells move by chemotaxis, the SDF1 would inhibit contractility at the front of the cluster, whilst the contractility at the back continued. Using laser ablation and optogenetics, we found that the contractility specifically at the rear of the cluster was driving the directed migration of the neural crest. And we could do that in vitro and in vivo. Of course when you say it, it sounds really obvious because rear contractility contributes to the driving forces of migration in cells, we’ve known that for years. But the novelty was that we had seen the whole cluster was acting like a single cell, where many cells at the front had a protrusion, and many cells at the back had a contraction, which we described as a supracell. And so, the analogy of how a single-cell moves was essentially expanded up to the scale of a cluster. We had this idea for quite a while, but we never had the techniques to address it. We did initially use blebbistatin and attempted to use mosaics, but those methods were very crude, so it was difficult to get any specific conclusions.
Can you tell us about your decision to stay with Roberto for your postdoc and how your research focus evolve during this time?
When I was in my completing research status (CRS) year, which is supposed to be your writeup year, I was struggling to finish off the paper and at the same time I had a deadline to submit my thesis. I was trying to get both of those done. I managed to get the paper submitted and then in for the revisions. Then I had about three or four weeks to write my thesis; I just wrote non-stop for about a month and got the thesis submitted! Then I think I had a round of revisions to do for the paper, so I had to stay on for a little bit longer to do those. Then I had my viva and by that point, it was November or December of that year, and I was just exhausted. I had not planned or considered my future at all at that point. I know you’re supposed to be looking for positions at least six months in advance, you can’t just ring someone up. So, at that point it was Christmas and Roberto offered that I could stay. The idea initially was just to stay for a little bit so that I could continue working until I found a postdoc position. I started my postdoc with Roberto basically a month later. Then, of course, the good thing about staying in a lab is that you already know how to do everything, so you can be super productive. But I did want to push my skillset, because many of the ideas I had required new techniques. So I developed some new methods especially in the context of labelling tissues in vivo and measuring and manipulating mechanics in vivo, as I was keen to explore what I saw was an open question of how chemical and mechanical cues interact in vivo. Fortunately, the lab acquired a nanoindenter to do mechanical measurements at around the same time. The combination of new techniques to address what I thought was a big question, and some promising results, led me to stay for the project.
Can you summarise the main findings from your recent paper?
There are a few main findings, one of them is that we saw durotaxis in vivo. Durotaxis is moving along a stiffness gradient, typically from soft substrates to a stiff substrate, which has been known for 22 years, but there was scarce evidence in vivo. So, that was the first one; we found that the neural crest undergoes durotaxis in vivo as well as chemotaxis, which we previously knew. And then following on from that, we found that the stiffness gradient was being formed by the neural crest cells themselves. The neural crest mechanically modifies an adjacent tissue, the placodes, and in doing so they generate a gradient in their own substrate. That was a very cool and surprising finding. And then towards the end of the paper, we describe how the mechanical signals in durotaxis and chemical signals in chemotaxis interact, how there’s interplay between those two. So essentially, both of these guidance cues work on the same set of proteins, Rho, Rac and actomyosin, influencing contractility. They work together in a cooperative manner. I think that this is going to be a big question for many systems in the next decade or so: how do the chemical signals and the mechanical ones interact to control various biological processes?
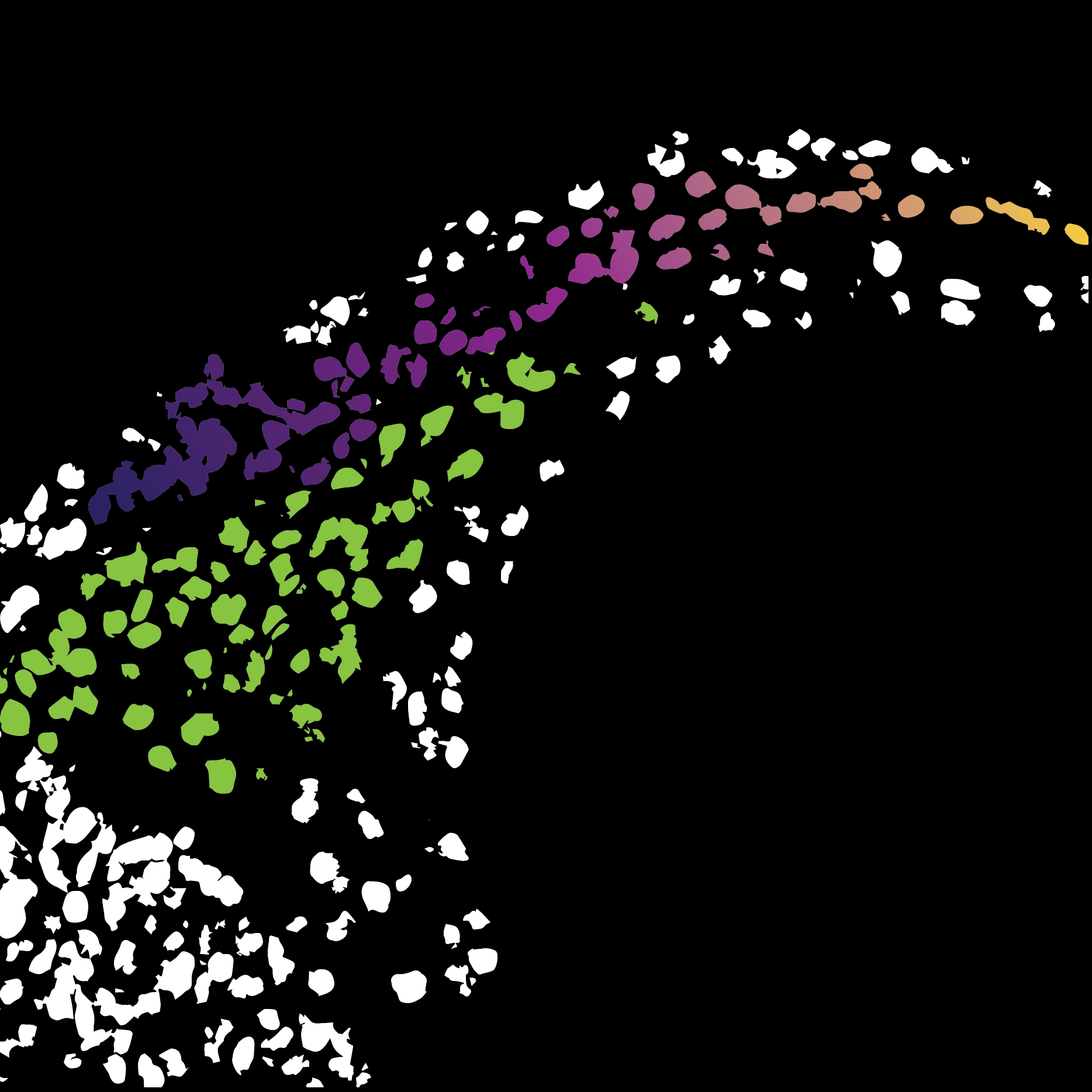
It’s interesting that the stiffness gradient moves with the cells.
Yes, so we had this result that there was a stiffness gradient. But at the time I was brand new to doing mechanical measurements, and as I was quickly learning, doing these measurements in embryos is really difficult. The embryos are super soft, which means that the cantilever you use also has to be really soft. All this means that even the tiniest thing can make a deflection and screw up your measurement; if it sticks, or if there’s a tiny piece of dust, anything like that. So, getting the data from the embryos was a really hard slog in the beginning. After we had observed the gradient, the obvious question is what happens at later time points when the cells move. We could have just seen that the gradient doesn’t move, that could totally make sense as well, the cells just move up the gradient. But, when we saw that the gradient moves, it was a very nice lab meeting slide!
During lockdown, you set up the Cell Migration webinar series, was this something you already had in mind or was it prompted by the pandemic? Can you tell us about the series and why you think it has been so successful?
Yes, the initiation of the series was totally pandemic driven. I don’t think anyone had even thought about virtual seminars pre-pandemic. I initially thought of the idea maybe a week into lockdown, but I didn’t act on it. After about a month or two, I started seeing other seminars pop up and people discussing them on Twitter. It seemed that people were interested in attending, because my initial worry was about putting all the effort in, and then having no one show up! So, it was good to see that people were attending virtual meetings on other topics. And whilst the series was pandemic driven, I’m really happy that it’s still going on. It’s been two years now and it’s still regularly getting high attendance, which is great. I guess it’s popular because people are interested in seeing seminars on their research topic. The cell migration community is a lot more diverse and vibrant than I’d previously known, so it is still attracting a lot of interest! The success is also due to Becky Jones, Jen Mitchell and Ankita Jha, who has taken over my role in organising the webinars, because it is quite a lot of effort.
Do you have any plans for in-person meetings linked to the series, or will you stick with the current format?
I’m not sure, I know some attendees have suggested that maybe the webinars could be organised as a one-day meeting for early career researchers in migration, which I think Jen and Ankita might be considering. But with the return of in-person meetings in cell migration, like the Abercrombie meeting this year and the GRC next year, I’m not sure whether adding another meeting would be a bit overkill. So, I think the virtual meetings will be there for now.
What’s next for you, both short term and longer term?
Short term, I’m trying to finish off a project for which I’m developing a lot of new skills for! I’m hoping to submit the paper before the end of the year, that is my optimistic plan. And then next year, I will be moving on to ‘destination unknown’. I’m considering my options, perhaps a short postdoc in another lab, or maybe a fellowship where you do a few months in many different labs, just to learn some new skills and experience some different environments before applying for positions. That’s one option that I’m considering, but I’m not totally sure yet.
It sounds like you are a big fan of developing new tools and techniques, is that something you enjoy doing?
I enjoy it, but it’s incredibly frustrating. I do it because I have to, not because I want to! I’m kind of attracted to the high risk, high reward projects, the projects that have a lot of potential. But often those are the projects that would have been done if the tools already existed. For example, the project I’m working on now, I’m forced to make new tools. But every time I do this, I always remember how difficult it is and how many months you have to spend developing these tools just to do a single experiment. So yes, I do it because I’m forced to, not because I want to; I enjoy using other people’s tools more than I enjoy making my own!
So, is it more that the question comes first and then you have to find a way to answer it, even if that means tool development?
Yes, that’s absolutely it. For example, in my postdoc I was interested in looking at the neural crest in vivo, in Xenopus, which as anyone who works with Xenopus knows, doing in vivo imaging is really, really difficult. There weren’t even any good antibodies for the neural crest; in the past it had always been inferred by the fact that there’s a fibronectin ECM around it. I spent a few months just developing fluorescence in situ hybridization for the neural crest so I could co-label it with other markers. So yes, the question always comes first, and then whatever technique I need to use to address it as best I can, that comes second.
What do you think the big questions in developmental biology will be over the next ten years?
One of the things that I think will be important, as I mentioned before, is the integration of mechanical and chemical cues, or signals or factors, in trying to understand the cell behaviour in a holistic way. I think that comparatively, we know a lot about signalling pathways and step-by-step processes that are occurring in cells, and now, we’re even getting a decent amount of data about how mechanics affects those processes. But I think in terms of combining them we haven’t even scratched the surface of how these cues come together. And it’s not a trivial thing to do, because trying to do manipulations of those various things without having unwanted side effects is really, really challenging. I think that’s going to be one of the main questions for the next 10 years of developmental biology.
When you’re not in the lab, what do you do for fun?
I enjoy painting, especially with oil paints. I’m really liking ‘Duolingo’ at the minute because I’m awful at languages. I also enjoy travelling, which is a rarity, but I’m happy to accept invitations!


 (2 votes)
(2 votes)