Assembling a “Stem Cell Zoo”
Posted by Jorge Lazaro, on 12 July 2023
One of the most fascinating observations that comes from comparing mammalian development is the difference in developmental tempo across species (Ebisuya & Briscoe, 2018). Mice and humans develop through a series of stereotypical events requiring conserved molecular pathways. Yet, embryogenesis takes around 60 days in humans and 20 days in mice. How mice generate similar-sized embryos containing the same structures as humans in only half the time remains unknown. Moreover, for other unconventional mammalian species, our knowledge is even more limited.
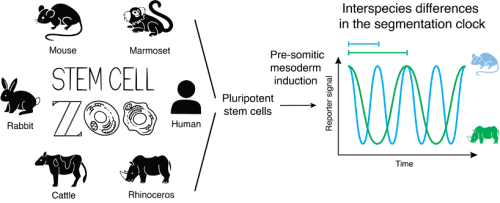
The Ebisuya lab has been addressing this question using the segmentation clock as a model system. The segmentation clock is the oscillatory gene expression found in the cells of the pre-somitic mesoderm (PSM) that controls the periodic formation of vertebrate body segments. These oscillations are cell-autonomous, and their period differs across species: around 30 min in zebrafish, 90 min in chicken, 100 min in snake, 2 hours in mouse and 5 hours in human (Matsuda et al, 2020b; Gomez et al, 2008). In our past study, in vitro recapitulation of the segmentation clock using mouse and human pluripotent stem cells (PSCs) revealed that differences in the biochemical reaction speeds, including protein degradation rates and gene expression delays, are responsible for the 2-3 times slower tempo of the human clock compared to that of the mouse (Matsuda et al, 2020a). However, whether this constitutes a general mechanism of developmental time control across mammals remained to be determined. In this study, we used PSCs to recapitulate in vitro the segmentation clock of four novel mammalian species in addition to the mouse and human: marmoset, rabbit, cattle and rhinoceros. We then used this “stem cell zoo” platform to systematically investigate the general mechanism behind the interspecies differences in developmental tempo (Lázaro et al, 2023).
The bigger the better?
Based on the results obtained comparing mouse and human developmental time, our first hypothesis was that the slower tempo of human was due to its bigger size. Several biological processes such as metabolic rates or gestation periods are known to scale with body weight. Larger animals tend to have slower metabolism, longer gestation, extended lifespan and scale most of their physical and biological properties to match their big size. Therefore, initially it made sense that developmental tempo could be regulated by similar rules. For this reason, to extend our zoo we wanted to have the largest mammal we could possibly get stem cells from. This animal turned out to be the southern white rhinoceros.
The question most people ask is: how did you manage to get rhinoceros cells? This is thanks to the work of Prof. Thomas B. Hildebrandt and colleagues in trying to save the northern white rhinoceros from extinction. For this, they have derived high quality embryonic stem cell lines of rhinoceros which can now be used for different studies (Hildebrandt et al, 2018). The first thing we did for this project was to obtain the rhinoceros stem cells and differentiate them into PSM. To our surprise, despite rhinoceros being much larger than human, their PSM cells showed a faster tempo. We then thought that maybe the slower tempo of human was due to a primate specific feature. Therefore, we searched the primate literature and found studies describing the very slow development of the common marmoset monkey. Common marmoset is a very small primate with a longer embryogenesis length than human. We obtained marmoset PSCs and, after differentiating them to PSM, we confirmed that their tempo was indeed slower than that of human. With the examples of rhinoceros and marmoset, it started to be evident that early developmental time could be uncoupled from the animal body weight, proving wrong our initial hypothesis.
We completed the zoo with rabbit and cattle cells to have a more complete phylogenetic representation of our species. Overall, our zoo contains species with adult body weights spanning from 50 grams to 2 tonnes, and gestation lengths ranging from 20 days to 17 months. These species belong to three distinct phylogenetic clades: Primates (marmoset and human), Glires (mouse and rabbit) and Ungulates (cattle and rhinoceros), constituting a diverse sampling of mammalian species unprecedented for developmental studies.
The stem cell zoo
Advancement in PSC technologies opens up new possibilities for broadening our understanding of mammalian development beyond traditional human and mouse models. The utilization of in vitro models representing various species poses a unique opportunity to conduct interspecies comparisons of cell- and tissue-autonomous processes (Figure 1). By recapitulating the segmentation clock of six mammalian species, we observed that the oscillatory period did not scale with the animal body weight but with the embryogenesis length. The biochemical kinetics of the core clock gene HES7 displayed clear scaling with the species-specific segmentation clock period. However, the cellular metabolic rates did not show an evident correlation. Instead, genes involving biochemical reactions showed an expression pattern that scales with the segmentation clock period.
A zoo of possibilities
In this study, we have focused on establishing correlations between developmental time and the different cellular parameters across species. In the future, we would like to test our hypothesis by establishing causal relationships between these processes, trying to better understand the genetic control of species-specific tempo establishment. Additionally, the stem cell zoo opens up possibilities to investigate a plethora of developmental processes across species. Other projects we have ongoing in the lab are the study of interspecies differences in brain development or heart beat rate determination. The use of stem cells allows us to study animals that are normally inaccessible in a lab but have particular features that make them interesting. We hope that the expansion of the stem cell zoo will spark further comparative studies across species.
Access the article
Jorge Lázaro, Maria Costanzo, Marina Sanaki-Matsumiya, Charles Girardot, Masafumi Hayashi, Katsuhiko Hayashi, Sebastian Diecke, Thomas B. Hildebrandt, Giovanna Lazzari, Jun Wu, Stoyan Petkov, Rüdiger Behr, Vikas Trivedi, Mitsuhiro Matsuda, Miki Ebisuya. – A stem cell zoo uncovers intracellular scaling of developmental tempo across mammals. Cell Stem Cell. 2023 Jul 6; 30: 938-949.e7
References
Ebisuya M & Briscoe J (2018) What does time mean in development? Dev 145
Gomez C, Özbudak EM, Wunderlich J, Baumann D, Lewis J & Pourquié O (2008) Control of segment number in vertebrate embryos. Nature 454: 335–339
Hildebrandt TB, Hermes R, Colleoni S, Diecke S, Holtze S, Renfree MB, Stejskal J, Hayashi K, Drukker M, Loi P, et al (2018) Embryos and embryonic stem cells from the white rhinoceros. Nat Commun 9: 1–9
Lázaro J, Costanzo M, Sanaki-Matsumiya M, Girardot C, Hayashi M, Hayashi K, Diecke S, Hildebrandt TB, Lazzari G, Wu J, et al (2023) A stem cell zoo uncovers intracellular scaling of developmental tempo across mammals. Cell Stem Cell 30: 938-949.e7
Matsuda M, Hayashi H, Garcia-Ojalvo J, Yoshioka-Kobayashi K, Kageyama R, Yamanaka Y, Ikeya M, Toguchida J, Alev C & Ebisuya M (2020a) Species-specific segmentation clock periods are due to differential biochemical reaction speeds. Science (80- ) 369: 1450–1455
Matsuda M, Yamanaka Y, Uemura M, Osawa M, Saito MK, Nagahashi A, Nishio M, Guo L, Ikegawa S, Sakurai S, et al (2020b) Recapitulating the human segmentation clock with pluripotent stem cells. Nature 580: 124–129


 (3 votes)
(3 votes)
interesting reading (have yet to read); enjoy!