Behind the paper: The mechanisms underlying microglial expansion in the developing brain
Posted by the Node, on 1 May 2023
Dr Liam Barry-Carroll, Dr David A Menassa, Professor Diego Gomez-Nicola and colleagues have recently published a paper in Cell Reports elucidating the mechanisms by which microglial cells expand as a population in the mouse brain using fate-mapping approaches. We asked Dr Barry-Carroll to give us a behind the scenes look into how the story came together.
How did you get started on the project?
I started working on the project when I joined the Gomez-Nicola lab in 2017 to start my PhD. I had been interested in studying microglia and so I applied for the position on a website called findaphd.com and was lucky to be accepted. For me it was interesting to study the cells in a healthy context which can be so often overlooked in the field.
What was already known about microglial colonisation and expansion in rodents?
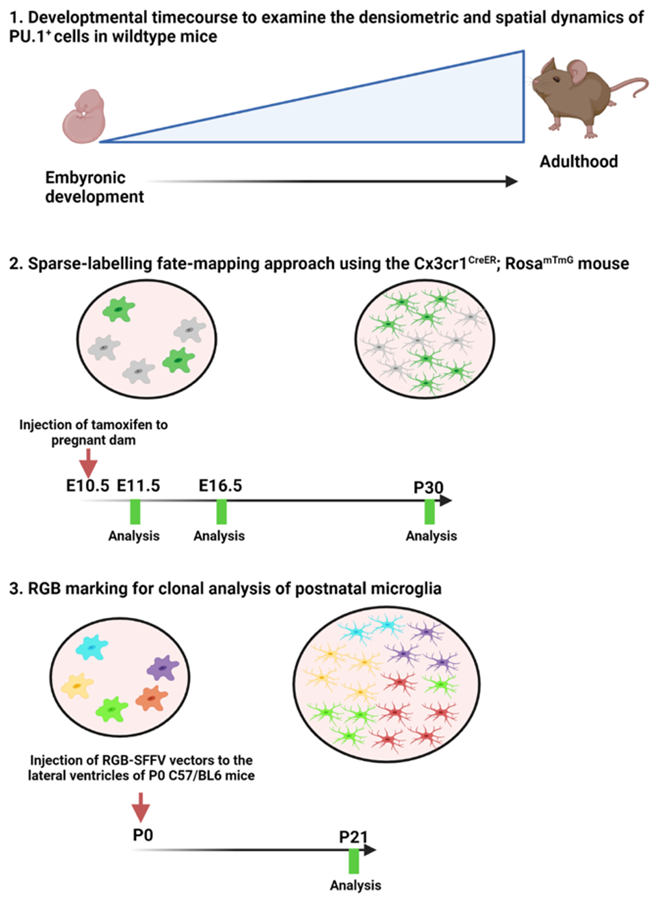
Studies coming out in the 1990s were able to demonstrate that progenitors of microglia were highly proliferative and subsequent studies had shown that a relatively small number of microglia progenitors go on to colonise the entire brain in just a short timespan during early postnatal life. However, it was unknown whether this was through clonal expansion or whether it was a more stochastic process of random proliferation of all the cells, as is the case of microglia in the healthy adult brain. Interestingly we can gain some insight from disease models whereby fate mapping studies have demonstrated that microglia will clonally expand in response to injury or disease. Our goal here was to see which of these potential mechanisms is responsible for the developmental colonisation of the brain by microglia.
Can you summarise your findings?
Here we were able to build on the findings of previous studies and showed that microglia expand quite rapidly, particularly during early development and that this expansion is correlated with the growth of the brain. As development continues, we could see changes in the spatiotemporal distribution of microglia from more dense clusters until late postnatal development (P21) when they formed a tiled or mosaic distribution allowing them to really cover the entire cortex and parenchyma. Using two methods of fate mapping, we demonstrated that microglial progenitors clonally expand during embryonic and postnatal development. Our multicolour lentiviral labelling approach allowed us to carry out clone-by-clone analysis and we observed that the mosaic of microglia is made up of inter-locking clones ranging in size from a couple of cells to quite large clones, indicating a disparity in the proliferative rate of different microglial progenitors during development. Subsequent mathematical modelling confirmed our finding that the proliferative potential is heterogenous among microglial progenitors. Another interesting finding was that microglia from larger clones tended to be spatially associated which may result in clonal dominance in certain brain regions.
When doing your research, did you have a eureka moment that has stuck with you?
For me, the moment came when I applied the spatial analysis to the different experimental setups, that is when we could clearly see the same spatial trends present in our different set-ups.
What about the flipside? Any moments of despair or frustration?
There were some moments of despair, particularly in the beginning when we were testing different multicolour reporters to much less avail. Eventually it came down to a promoter that was not efficiently expressed in microglia. We managed to overcome this hurdle by setting up the sparse-labelling protocol as suggested by Dr Salah Elias (University of Southampton) who is a developmental scientist.
Where will this story take you next?
For now, I have finished this project and started a postdoc in the Nutrineuro laboratory in Bordeaux, France. However, I cannot say that I wouldn’t like to revisit this topic in the future, and I hope that our study will inspire some more research into this area, particularly in understanding the molecular mechanisms involved in the regulation of microglial proliferation and the potential sources of this proliferative heterogeneity.
What is next for you after this paper?
As I said, I have recently started my journey as a postdoc with Dr Jean-Christophe Delpech and Dr Sophie Layé and I am applying my knowledge of microglia in the field of extracellular vesicles. I am really looking forward to seeing how I can combine these different research topics and all that I have learned and ultimately build my future research career. Exciting times ahead!


 (No Ratings Yet)
(No Ratings Yet)