BSDB Gurdon Studentship Report – Rihova
Posted by Laura Rihova, on 15 December 2022
Determining the Effects of FOXG1 Mutations on Early Neurodevelopmental Structures Using iPSCs


I am an undergraduate Neuroscience student at University College London interested in researching neurodevelopmental and neuropsychiatric disorders. I am grateful for the opportunity that the Gurdon grant gave me to undertake a summer studentship in the lab of Dr Srinjan Basu at the Wellcome-MRC Cambridge Stem Cell Institute under the supervision of Dr Deep Adhya. The lab focuses on imaging organoids to study how chromatin regulators influence stem cell differentiation in the neurodevelopmental conditions autism and epilepsy.
iPSCs and brain organoids as a model for neurodevelopmental conditions
The central nervous system develops from a monolayer of neuroepithelial cells which folds to form the neural tube. Neural stem cells organise themselves within the tube to form neural rosettes, rose-like structures which have been reported both in vivo and in vitro (Hříbková et al. 2018). Recent advances in in vitro neuronal differentiation and organoid technology provide a model system for addressing how normal development of these rosettes is disrupted in conditions such as autism or epilepsy and for dissecting the molecular mechanisms governing these changes. Induced pluripotent stem cells (iPSCs), adult somatic cells reprogrammed back into their pluripotent stem cell stage, can be induced to differentiate into specific cell fates (Fig.1). Using this approach, it has been shown that iPSCs generated from individuals with autism show significant cellular and molecular abnormalities (Adhya et al. 2021). Intriguingly, defects begin much earlier than expected. Autistic iPSCs form abnormal neural rosette structures long before neural stem cells differentiate towards excitatory/inhibitory neurons (Adhya et al. 2021), but how this occurs at the molecular level remains poorly understood.
My project: FOXG1, a transcription factor implicated in epilepsy and autism
The aim of my project was to determine if atypical neural rosette structures form in early cortical organoids from FOXG1-mutant iPSCs. FOXG1 is an essential transcription factor responsible for normal neurodevelopment. Mutations in FOXG1 are significantly associated with syndromic forms of autism and ~90% of FOXG1 syndrome patients show epileptic seizures (Seltzer et al. 2014). Therefore, autism and epilepsy are considered comorbid.
Methods and techniques learned
During my project, I learned how to perform tissue culture (TC): I grew iPSCs and differentiated them into neurons. Working in the sterile TC hood made me a better scientist as I became more conscious about ways to prevent contamination, which is especially important when handling live cell cultures.
After successfully growing the iPSCs, I fixed them before differentiation and then at several stages during neural rosette formation (Fig. 1). The fixed cells were processed for immunofluorescence (IF) imaging and quantitative polymerase chain reaction (qPCR) to determine morphological changes that take place during cortical differentiation and to see its effect on gene expression, respectively. I isolated RNA using the Mini prep kit, but unfortunately, was unable to continue with the qPCR as some essential components did not arrive in time. Nevertheless, we got interesting results from the imaging alone (Fig. 2). Immunocytochemistry staining was another important lab skill I gained during the studentship. After surveying the current literature, I chose antibodies that reveal morphological features of neural rosettes: SOX1 as an early differentiation marker, PAX6 as a well-established marker of cortical neurons, N-cadherin as a rosette lumen marker, and TUBB3 as a neural cytoskeletal marker. Additionally, DAPI was used as nuclear counterstain.
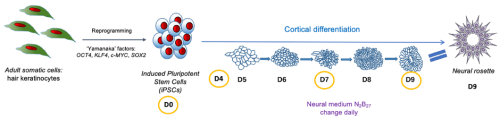
Fig. 1 shows the outline and timeline of my project. iPSCs were generated by reprogramming adult somatic cells with ‘Yamanaka factors’ into pluripotent stem cells. The iPSCs were subsequently differentiated into neurons by placing them in a neural medium. The cells were fixed and subjected to IF at day 0, 4, 7 and 9 of the cortical differentiation. Neural rosettes form at day 9.
Apart from learning new lab techniques, I was introduced to image analysis softwares such as Fiji and CellProfiler. Furthermore, I was fully immersed into the activities of the research group, including weekly journal club, group meetings and workshops. I had several presentations during the group meetings which helped me improve my ability to discuss scientific concepts and results. All these skills will be useful for the rest of my MSci degree and for a future PhD.
Project outcomes
iPSCs stained with N-cadherin and SOX1 (Fig.2A) or with TUBB3 and PAX6 (Fig.2B) revealed a phenotype for the FOXG1 mutation. Zooming in on the rosette lumen stained with N-cadherin and nuclei stained with DAPI (Fig.2C) showed that neural rosettes do not form at day 9 without FOXG1 while they form in control iPSCs. Interestingly, N-cadherin, PAX6 and TUBB3 stainings reveal that FOXG1-mutant cells at day 9 look like glial progenitor cells, precursors that should form later in the organoid. Altogether, these findings provoke numerous questions about underlying mechanisms of these morphological changes, effect on gene expression and further differentiation into excitatory/inhibitory neurons, that will be researched by the lab in the future. If I had more time in the lab, I would have looked into other gene mutations significant for the comorbidity of autism and epilepsy that may affect the formation of neural rosettes, namely KMT2A and FGFR2. That would help us get a more robust understanding of the shared neurodevelopmental defects of these disorders.
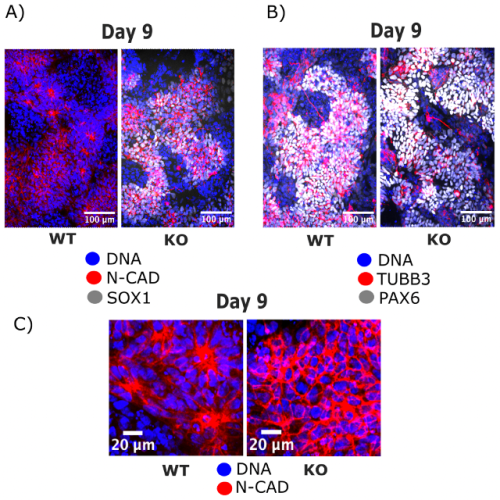
Fig. 2: IF imaging of iPSCs at day 9 of cortical differentiation shows the disruption in neural rosette formation that occurs in FOXG1-mutant cells (KO) while neural rosettes develop normally in control cells (WT).
Summary
Overall, the summer studentship was an amazing experience as I designed my own experiments that I saw through from the beginning to the end. Along the way I gained valuable skills in the lab and research in general. This project introduced me to the exciting field of organoid research that is redefining the way we study neurodevelopmental disorders, and I would like to continue with it in my scientific future. I would strongly recommend applying for the Gurdon grant to students interested in delving deeper into a developmental biology topic and in experiencing what being a scientist entails.


 (1 votes)
(1 votes)
Great summary Laura. I really enjoyed reading about what you did