Cellularization in Drosophila, University of Chicago Journal Club
Posted by UChicagoDRSB_JC, on 15 March 2014
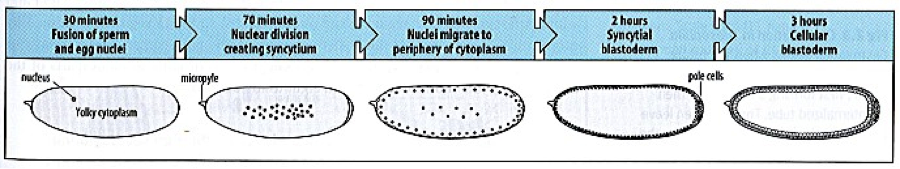
Cellularization in Drosophila embryos is quite the remarkable process. After fertilization, nuclear division occurs rapidly but without cell membrane formation, leading to a syncytial embryo with many nuclei in a common cytoplasm. After 9 mitotic divisions, the nuclei begin migration to the periphery of the syncytium, such that nuclei line the plasma membrane of the embryo. Cellularization, the process that creates an individual cell membrane for each nucleus, then begins at cell cycle 14. This process occurs as a simultaneous ingression of membrane around each nucleus to build a sheet of epithelial cells (fig. 1).
The cellularization process occurs over the course of about an hour, and the membrane surface area increases some 25-fold (Lecuit and Wieschaus, 2000). As a result, this time period is marked by a huge need for new membrane.
So where is this membrane coming from? It is known that the plasma membrane of the syncytial Drosophila embryo is covered in microvilli – small, finger-like protrusions of membrane. After cellularization, the microvilli are no longer present. Early researchers proposed that the membrane needed for cellularization could be “stored” in these villi, and the flattening of the structures led to increased membrane surface area (Fulllilove and Jacobson, 1971). However, little experimental evidence exists to support this model of membrane origin.
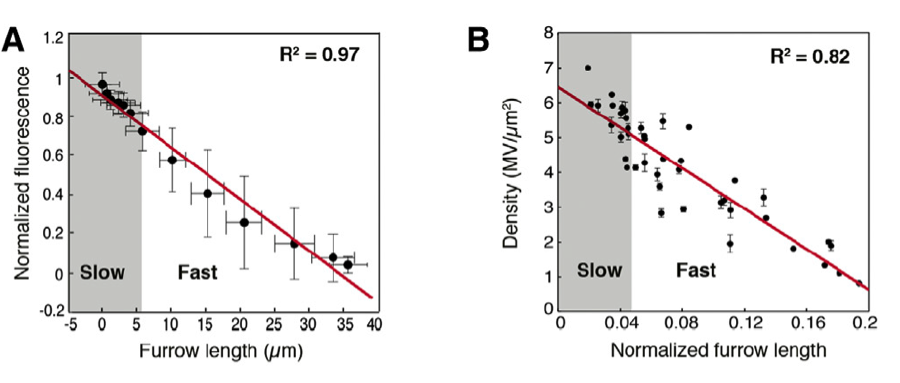
A recent paper in Developmental Cell approaches the question again. Figard and colleagues (2013) examine the role of microvilli during cellularization by investigating whether the rate at which microvilli are depleted can be correlated with the rate at which cell membranes are forming around each nucleus. Using live imaging and time-lapse techniques, the authors found that the depletion of microvilli was coupled with cell membrane furrow ingression during cellularization (fig. 2). This result strongly implies microvilli do in fact act as a source of membrane during Drosophila cellularization.
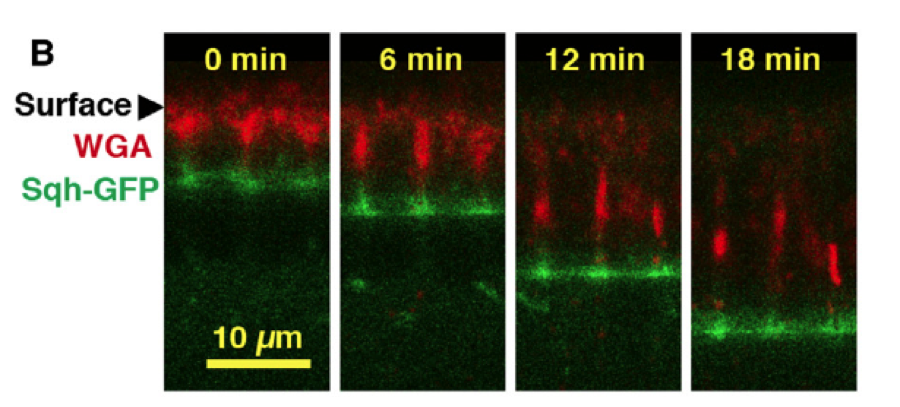
This result raises the question as to how the microvillar membrane is incorporated into the cell membrane: is it endocytosed from the apical surface and deposited elsewhere or is it pulled directly into the furrow, i.e. the cell straightens out the membrane? Using pulse-chase time-lapse imaging, the authors followed the redistribution of the microvillar membrane over time and found that it is pulled directly into the membrane furrow as cellularization proceeds (fig. 3).
Figard and colleagues (2013) provide new evidence for an old idea, and set forth a model for cellularization in which the ingressing membrane pulls microvilli into the furrow, thereby utilizing a large amount of previously folded membrane. This work unifies current ideas about cellularization, providing a beautiful overview of this complex embryonic process. For instance, the authors propose exocytosis of organelle membrane to the apical surface, as shown in Lecuit and Wiechaus (2000), contributes to the flow of membrane into the ingressing furrows. The paper effectively outlines the kinematics of this process, including a two-stage progression of cellularization.
For the whole story access the paper here.
References
Figard, L., Xu, H., Garcia, H.G., Golding, I., Sokac, A.M. The Plasma Membrane Flattens Out to Fuel Cell-Surface Growth during Drosophila Cellularization. (2013) Developmental Cell, 27 ( 6), pp. 648-655.
Fullilove, S.L., Jacobson, A.G. Nuclear elongation and cytokinesis in Drosophila Montana. (1971) Developmental Biology, 26 (4), pp. 560-577.
Lecuit, T., Wieschaus, E. Polarized insertion of new membrane from a cytoplasmic reservoir during cleavage of the Drosophila embryo. (2000) Journal of Cell Biology, 150 (4), pp. 849-860.
This post results from the discussion of Figard et al., 2013 by the Development, Regeneration, and Stem Cell Biology Journal Club at the University of Chicago. It was authored by Haley K. Stinnett, Department of Organismal Biology and Anatomy, University of Chicago, Chicago, IL 60637.


 (4 votes)
(4 votes)