In Development this week (Vol. 143, Issue 12)
Posted by Seema Grewal, on 14 June 2016
Here are the highlights from the current issue of Development:
A distinct cartilage programme for bone regeneration

Bone healing, for example fracture repair in humans, often involves a cartilage intermediate but how this tissue is induced and contributes to healing is unclear. Here, Gage Crump and co-workers show that regeneration of the zebrafish jawbone involves cells of a hybrid cartilage-bone nature (p. 2066). They first report that the lower jawbone of adult zebrafish regenerates via a cartilage intermediate. The analysis of cells within this injury-induced cartilage reveals that they express both chondrocyte- and osteoblast-associated genes and can undergo mineralization. This is in contrast to the situation observed in developmental chondrocytes of zebrafish, which do not express osteoblast genes and do not mineralize. The researchers further report that these repair chondrocytes likely arise from the periosteum – a tissue that usually gives rise to osteoblasts. Finally, they demonstrate that the induction of repair chondrocytes from the periosteum involves an unexpected role for Indian hedgehog signalling, which is normally involved in chondrocyte proliferation during development. Thus, while it has generally been assumed that regeneration involves the same processes that are employed during development, this study suggests that regeneration induces a unique cartilage differentiation and repair programme.
Insights into cadherin function in the neocortex

Development of the mammalian neocortex involves the radial migration of neurons, which move from their place of birth to their final position in the appropriate neocortical cell layer. This migration is known to involve cadherins but the specific cadherins implicated and the mechanisms by which they act are unclear. Now, on p. 2121, Ulrich Mueller and colleagues report that cadherin 2 (CDH2) and cadherin 4 (CDH4) play crucial roles during radial neuronal migration in the mouse neocortex. The researchers first demonstrate that both CDH2 and CDH4 are expressed in the developing mouse neocortex. The inactivation ofCdh2 or Cdh4 specifically in migrating neurons reveals that both are required for radial migration. The authors further report that CDH2 and CDH4 act via protein tyrosine phosphatase 1B (PTP1B) and α- and β-catenins to control migration. Finally, they show that the perturbation of cadherin-mediated signalling has no effect on the formation or extension of neuronal leading processes but instead disrupts nucleokinesis – the process by which the nucleus translocates forward during migration. These and other findings suggest that cadherin-mediated signalling to the cytoskeleton is crucial for radial migration in the neocortex.
Germ cell migration: as easy as ABC?

The development of the Drosophila embryonic gonad requires the migration of primordial germ cells (PGCs) towards somatic gonadal precursors (SGPs). Previous studies have implicated a role for the ATP-binding cassette (ABC) transporter Mdr49 during this event, suggesting that it functions in the export of a PGC attractant. Here, Girish Deshpande and co-workers further explore the function of Mdr49 in flies (p. 2111). They report that Mdr49 mutant embryos exhibit PGC migration defects but that these can be alleviated by a cholesterol-rich diet. Given that cholesterol is known to be involved in Hedgehog (Hh) precursor protein processing, the authors explore the potential link between Hh signalling and PGC migration. Their studies demonstrate genetic interactions between Mdr49 and genes encoding Hh pathway components, both during PGC migration and wing development. Importantly, the authors reveal that Hh release from hh-expressing cells is compromised in Mdr49 mutant embryos. Overall, these findings highlight a role for Mdr49 in the Hh pathway and lead the authors to propose that Mdr49 functions to allow SGPs to produce sufficient amounts of processed Hh that, in turn, signals to guide migrating PGCs.
PLUS…
Ten years of induced pluripotency: from basic mechanisms to therapeutic applications
 Ten years ago, the discovery that mature somatic cells could be reprogrammed into induced pluripotent stem cells (iPSCs) redefined the stem cell field and brought about a wealth of opportunities for both basic research and clinical applications. To celebrate the tenth anniversary of the discovery, the International Society for Stem Cell Research (ISSCR) and Center for iPS Cell Research and Application (CiRA), Kyoto University, together held the symposium ‘Pluripotency: From Basic Science to Therapeutic Applications’ in Kyoto, Japan. Here, summarize the main findings reported as well as the enormous potential that iPSCs hold for the future. See the Meeting Review on p. 2039
Ten years ago, the discovery that mature somatic cells could be reprogrammed into induced pluripotent stem cells (iPSCs) redefined the stem cell field and brought about a wealth of opportunities for both basic research and clinical applications. To celebrate the tenth anniversary of the discovery, the International Society for Stem Cell Research (ISSCR) and Center for iPS Cell Research and Application (CiRA), Kyoto University, together held the symposium ‘Pluripotency: From Basic Science to Therapeutic Applications’ in Kyoto, Japan. Here, summarize the main findings reported as well as the enormous potential that iPSCs hold for the future. See the Meeting Review on p. 2039
Phosphoinositide signaling in plant development
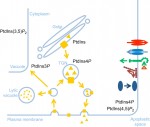 The membranes of eukaryotic cells create hydrophobic barriers that control substance and information exchange between the inside and outside of cells and between cellular compartments. Besides their roles as membrane building blocks, some membrane lipids, such as phosphoinositides (PIs), also exert regulatory effects. Indeed, emerging evidence indicates that PIs play crucial roles in controlling polarity and growth in plants. Here, Ingo Heilmann highlights the key roles of PIs as important regulatory membrane lipids in plant development and function. See the Primer article on p. 2044
The membranes of eukaryotic cells create hydrophobic barriers that control substance and information exchange between the inside and outside of cells and between cellular compartments. Besides their roles as membrane building blocks, some membrane lipids, such as phosphoinositides (PIs), also exert regulatory effects. Indeed, emerging evidence indicates that PIs play crucial roles in controlling polarity and growth in plants. Here, Ingo Heilmann highlights the key roles of PIs as important regulatory membrane lipids in plant development and function. See the Primer article on p. 2044
Extracellular matrix motion and early morphogenesis
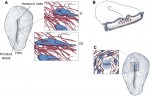 For over a century, embryologists who studied cellular motion in early amniotes generally assumed that morphogenetic movement reflected migration relative to a static extracellular matrix (ECM) scaffold. However, as Charles Little and colleagues discuss here, recent investigations reveal that the ECM is also moving during morphogenesis. See the Review article on p. 2056
For over a century, embryologists who studied cellular motion in early amniotes generally assumed that morphogenetic movement reflected migration relative to a static extracellular matrix (ECM) scaffold. However, as Charles Little and colleagues discuss here, recent investigations reveal that the ECM is also moving during morphogenesis. See the Review article on p. 2056


 (No Ratings Yet)
(No Ratings Yet)