In Development this week (Vol. 143, Issue 3 )
Posted by Seema Grewal, on 2 February 2016
Here are the highlights from the current issue of Development:
New insights into human neural crest induction

Human neural crest (NC) cells are the precursors for a wide range of ectodermal and mesenchymal cell derivatives. Due to the inherent difficulties associated with early human embryonic studies, it is currently unclear exactly when NC cells are specified and from what. Differentiation of human embryonic stem cells represents an excellent in vitro system to study NC induction; however, current protocols often involve cell conglomerates and/or undefined media, making it difficult to tease apart the key signalling events that drive induction. Now, on p. 398, Martín García-Castro and colleagues report a highly efficient, defined protocol for human NC induction and use this system to identify a population of cells, which they call ʻpre-neural plate borderʼ cells, that represent the earliest known stage of NC specification. WNT-induced molecular markers are used to characterise this population, which the authors show is specified distinct from ectoderm and mesodermal fates. The authors further demonstrate a crucial role for WNT/β-catenin signalling, as well as the dynamic effects of BMP and FGF signalling, in NC induction. These results support the in vivo origin of NC cells from non-neural progenitors and provide strong evidence for the key role played by WNT/β-catenin in human NC induction.
Mouse villification tells a different story

Coordination of villus formation during embryonic development is essential for the correct formation and function of the adult intestine. In the mouse, villus emergence is preceded by the formation of mesenchymal signalling clusters, but what triggers these mesenchymal clusters to form in a precise pattern is currently unclear. Recent data from the chick suggest that mechanical forces are at play, but whether this applies to mammalian species has not yet been addressed. Now, on p. 427, Deborah Gumucio and colleagues report that, unlike in chick, mouse villification is not patterned by mechanical forces but instead is driven by Bmp signalling. The authors demonstrate how different patterns of cluster distribution and villus outgrowth can be generated simply by changing the concentration of Bmp ligands or Bmp modulators that are expressed by the clusters, or by altering Bmp signal transduction. Indeed, epithelial deformation follows cluster formation in the mouse, whereas the opposite is true in the chick, highlighting the distinct villus patterning processes in the two species. Interestingly, the authors use mathematical modelling to demonstrate that their findings are consistent with Turing field patterning, adding mammalian villus formation to the list of Turing system-patterned developmental processes.
Axon guidance in good form(in)

In order to reach their targets, neurons must send out axons that navigate their environment until their final destination is reached. Although it is clear that this process involves extensive cytoskeletal remodelling within the growth cone filopodia at the axon tip, the mechanism by which this occurs remains poorly understood. In this issue (see p. 449), Aurnab Ghose and colleagues identify a novel role for formin 2 (Fmn2) in regulating filopodial dynamics by stabilising filopodial tip adhesions without affecting initiation or elongation. The authors show that Fmn2 colocalises with actin within growth cone filopodia of the developing chick spinal cord. Using Fmn2 and control morpholinos, the authors analyse the function of Fmn2 within the growth cone, with specific attention to its actin organisation, motility, substrate attachments and filopodial dynamics. The authors also demonstrate that Fmn2 is required cell-autonomously by spinal neurons for midline guidance and pathfinding in vivo. Finally, in non-neuronal fibroblasts, Fmn2 is shown to regulate the development of ventral stress fibres, in turn modulating the stability of focal adhesions. This work identifies Fmn2 as an important new regulator of cell adhesion and motility in growth cones and is suggestive of similar functions in non-neuronal cells.
A twist in the tail for Hoxb6

Hox genes typically display a high level of functional specificity during the formation of the vertebrate axial skeleton. To date, most studies have focused on understanding the mechanism responsible for Hox functional specificity, and thus little is known regarding the potential function of Hox proteins beyond their well-established roles. In this issue (p. 437), Moisés Mallo and colleagues uncover an interesting role for Hoxb6, distinct from its involvement in rib formation. The authors show that forced expression of Hoxb6 in the paraxial mesoderm produces non-rib-related malformations due to problems in somitogenesis and anterior-posterior somite patterning, which result from dysregulated expression of the oscillator gene Lfng. Dysregulated Lfng expression was restricted to regions posterior to the hindlimb, suggesting that the mechanisms of paraxial segmentation are not uniform along the main body axis as previously thought. These data provide a mechanistic connection between Hoxb6 expression and the mammalian segmentation clock and convincingly demonstrate functional differences in somitogenesis before and after the trunk-to-tail transition. The authors postulate that their data suggest the existence of yet-to-be-identified differential mechanisms operating during development of the trunk or tail areas of the body axis.
PLUS…
Hedgehog signalling
 The Hedgehog (Hh) signalling pathway is one of the key regulators of metazoan development. Here, provide an outline of the current understanding of Hh signalling mechanisms, highlighting the similarities and differences between species. See the Development at a Glance poster article on p. 367
The Hedgehog (Hh) signalling pathway is one of the key regulators of metazoan development. Here, provide an outline of the current understanding of Hh signalling mechanisms, highlighting the similarities and differences between species. See the Development at a Glance poster article on p. 367
The force within: endocardial development, mechanotransduction and signalling during cardiac morphogenesis
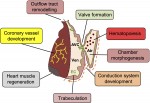 Endocardial cells are cardiac endothelial cells that line the interior of the heart tube. Here, discuss how the endocardium develops, how endocardial-myocardial interactions influence heart development, and how the dysregulation of blood flow-responsive endocardial signalling can result in pathophysiological changes. See the Review on p. 373
Endocardial cells are cardiac endothelial cells that line the interior of the heart tube. Here, discuss how the endocardium develops, how endocardial-myocardial interactions influence heart development, and how the dysregulation of blood flow-responsive endocardial signalling can result in pathophysiological changes. See the Review on p. 373
View from the heart: cardiac fibroblasts in development, scarring and regeneration
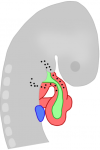 Nadia Rosenthal and co-workers discuss the role of cardiac fibroblasts in scarring and regeneration, as well as how these cells are specified during development and the unique characteristics that define them. See the Review on p. 387
Nadia Rosenthal and co-workers discuss the role of cardiac fibroblasts in scarring and regeneration, as well as how these cells are specified during development and the unique characteristics that define them. See the Review on p. 387


 (No Ratings Yet)
(No Ratings Yet)