In Development this Week (Vol. 144, Issue 15)
Posted by the Node, on 1 August 2017
Here are the highlights from the current issue of Development:
X-citing insights into dosage compensation
The non-coding RNA Xist plays a key role in the process of X chromosome inactivation (XCI) and is thus essential for dosage compensation of X-linked genes in females. 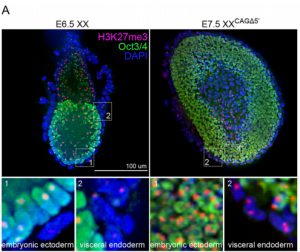 The 5′ region of Xist RNA contains a conserved element termed the A-repeat that is required for the silencing function of Xist in embryonic stem cells, but how this region functions during development is unclear. Now, Takashi Sado and co-workers explore this by introducing into mice a mutated Xist allele that produces Xist RNA lacking the A-repeat region (p. 2784). They first report that imprinted XCI is compromised upon paternal transmission of this allele. The authors further show that the mutant form of Xist is able to coat the X chromosome but fails to silence it in embryonic and extraembryonic tissues. Surprisingly, however, mutant Xist RNA is still able to silence a subset of genes in the trophoblast. Finally, the authors reveal that the failure of imprinted XCI has a more significant impact on genome-wide gene expression than expected; changes in the expression of both X-linked and autosomal genes are observed. Together these findings provide new insights into Xist-mediated gene silencing but also raise the intriguing possibility that dosage compensation regulates X-linked genes as well as gene expression more globally.
The 5′ region of Xist RNA contains a conserved element termed the A-repeat that is required for the silencing function of Xist in embryonic stem cells, but how this region functions during development is unclear. Now, Takashi Sado and co-workers explore this by introducing into mice a mutated Xist allele that produces Xist RNA lacking the A-repeat region (p. 2784). They first report that imprinted XCI is compromised upon paternal transmission of this allele. The authors further show that the mutant form of Xist is able to coat the X chromosome but fails to silence it in embryonic and extraembryonic tissues. Surprisingly, however, mutant Xist RNA is still able to silence a subset of genes in the trophoblast. Finally, the authors reveal that the failure of imprinted XCI has a more significant impact on genome-wide gene expression than expected; changes in the expression of both X-linked and autosomal genes are observed. Together these findings provide new insights into Xist-mediated gene silencing but also raise the intriguing possibility that dosage compensation regulates X-linked genes as well as gene expression more globally.
Non-neural roles for acetylcholinesterase
Acetylcholinesterase (AChE) is a highly conserved protein that is known for its essential role in degrading the neurotransmitter acetylcholine at neural synapses. However, it is expressed more broadly, outside the nervous system, suggesting that it may carry out additional functions. 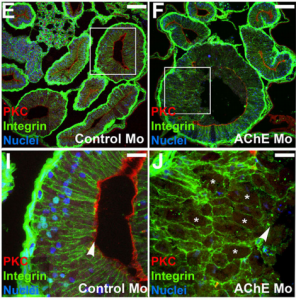 Now, on p. 2764, Nanette Nascone-Yoder and colleagues reveal that AChE plays an essential non-classical role in Xenopus gut morphogenesis. By exposing tailbud stage Xenopus embryos to AChE inhibitors, or by injecting embryos with morpholinos to knock down AChE in the intestinal endoderm, they show that AChE is required for proper intestinal morphogenesis; in the absence of AChE function, intestines are short/malrotated and exhibit a disorganised epithelium. This function of AChE , they report, is independent of its cholinesterase activity. Further analyses demonstrate that AChE is required for endoderm cell rearrangement and polarisation – events that drive gut lengthening and morphogenesis – as well as endoderm cell differentiation. Finally, the researchers demonstrate that AChE regulates cell-substrate but not cell-cell adhesion. Overall, these results provide direct in vivo evidence for a morphogenetic function for AChE in non-neuronal tissues and suggest that AChE may function in other aspects of development and physiology, a find that has important implications given the widespread use of cholinesterase inhibitors in the treatment of human diseases.
Now, on p. 2764, Nanette Nascone-Yoder and colleagues reveal that AChE plays an essential non-classical role in Xenopus gut morphogenesis. By exposing tailbud stage Xenopus embryos to AChE inhibitors, or by injecting embryos with morpholinos to knock down AChE in the intestinal endoderm, they show that AChE is required for proper intestinal morphogenesis; in the absence of AChE function, intestines are short/malrotated and exhibit a disorganised epithelium. This function of AChE , they report, is independent of its cholinesterase activity. Further analyses demonstrate that AChE is required for endoderm cell rearrangement and polarisation – events that drive gut lengthening and morphogenesis – as well as endoderm cell differentiation. Finally, the researchers demonstrate that AChE regulates cell-substrate but not cell-cell adhesion. Overall, these results provide direct in vivo evidence for a morphogenetic function for AChE in non-neuronal tissues and suggest that AChE may function in other aspects of development and physiology, a find that has important implications given the widespread use of cholinesterase inhibitors in the treatment of human diseases.
A chemical reset for pluripotency
Pluripotency in mammalian stem cells is thought to pass through two phases – an initial naïve phase and a later primed phase, mimicked in vitro by mouse embryonic stem cells and human pluripotent stem cells (hPSCs), respectively. Much effort has gone into converting hPSCs to a more naïve state, but current methods are not always reliable or broadly applicable across cell lines.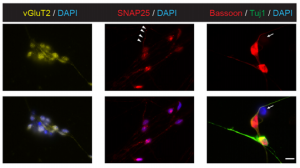 On p. 2748, Ge Guo, Austin Smith and colleagues provide a simple and efficient method for resetting human pluripotency based on transient inhibition of histone deacetylases (HDACs) with chemical inhibitors. HDAC inhibition leads to increased expression of naïve markers in a variety of different hPSC lines, and cells can be expanded in naïve culture conditions without requiring feeders. Chemically reset cells show a marked transcriptional difference to primed hPSCs and a similarity to epiblast cells of the preimplantation inner cell mass. Reset cells undergo global reduction in DNA methylation, have two active X chromosomes, and can differentiate into multiple lineages. This work provides a protocol for efficient resetting of hPSC pluripotency, and a transcription and methylation resource for further interrogation of the human naïve state.
On p. 2748, Ge Guo, Austin Smith and colleagues provide a simple and efficient method for resetting human pluripotency based on transient inhibition of histone deacetylases (HDACs) with chemical inhibitors. HDAC inhibition leads to increased expression of naïve markers in a variety of different hPSC lines, and cells can be expanded in naïve culture conditions without requiring feeders. Chemically reset cells show a marked transcriptional difference to primed hPSCs and a similarity to epiblast cells of the preimplantation inner cell mass. Reset cells undergo global reduction in DNA methylation, have two active X chromosomes, and can differentiate into multiple lineages. This work provides a protocol for efficient resetting of hPSC pluripotency, and a transcription and methylation resource for further interrogation of the human naïve state.
Morphogen signalling: Dpp gets Irked
Mutations in genes encoding ion channels cause severe defects in development across species but the underlying mechanisms, particularly in tissues other than neurons and muscle, have remained unclear.  On p. 2771, Emily Anne Bates and colleagues describe a role for Irk2, an inwardly rectifying potassium channel in Drosophilaorthologous to Kir2.1 in vertebrates, in regulating the release of the BMP family morphogen Dpp during development of the wing epithelium. Building on their previous finding that a dominant-negative Irk2 reduces Dpp signalling and causes wing defects, they now show that human KIR2.1 can substitute for inhibited DrosophilaIrk2, and that Irk2’s role is restricted to the cells that produce, rather than just transduce, the Dpp signal. Surprisingly, inhibiting Irk2 broadens the distribution of Dpp in the wing, but also alters the dynamics of Dpp release from cells, suggesting that Irk2 controls the timing of Dpp secretion. Irk2 inhibition reduces the amplitude of calcium spikes in wing cells, and depolarising the membrane with extracellular potassium leads to an overall increased release of Dpp, implicating Irk2’s regulation of membrane potential in Dpp release. Together, these results suggest that ion channels influence tissue morphogenesis by regulating the release of morphogens from the membrane.
On p. 2771, Emily Anne Bates and colleagues describe a role for Irk2, an inwardly rectifying potassium channel in Drosophilaorthologous to Kir2.1 in vertebrates, in regulating the release of the BMP family morphogen Dpp during development of the wing epithelium. Building on their previous finding that a dominant-negative Irk2 reduces Dpp signalling and causes wing defects, they now show that human KIR2.1 can substitute for inhibited DrosophilaIrk2, and that Irk2’s role is restricted to the cells that produce, rather than just transduce, the Dpp signal. Surprisingly, inhibiting Irk2 broadens the distribution of Dpp in the wing, but also alters the dynamics of Dpp release from cells, suggesting that Irk2 controls the timing of Dpp secretion. Irk2 inhibition reduces the amplitude of calcium spikes in wing cells, and depolarising the membrane with extracellular potassium leads to an overall increased release of Dpp, implicating Irk2’s regulation of membrane potential in Dpp release. Together, these results suggest that ion channels influence tissue morphogenesis by regulating the release of morphogens from the membrane.
PLUS…
Editorial changes
Development announces changes to the editorial team and invites community input in choosing a new Editor in Chief and in suggesting future areas for the journal to explore

An interview with Jim Smith
Jim Smith, recently knighted for services to medical research and science education, talks about his research career and his hopes for the future of biomedical science.
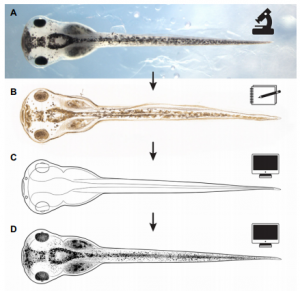
The Zahn drawings
New, freely available illustrations of developing Xenopus laevis, drawn by Natalya Zahn, provide a resource for teaching and research, especially in the field of craniofacial biology.
Programming and reprogramming the brain: a meeting of minds in neural fate
This Meeting Review discusses recent progress in our understanding of neuronal programming, highlighting some of the common features of cell fate determination during development and directed reprogramming.
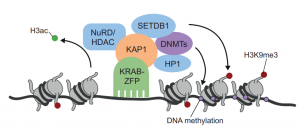
KRAB zinc finger proteins
This Primer summarises our current understanding of the intriguing family of KRAB-ZFP transcriptional regulators and its contribution to the control, evolution and co-option of transposable elements.


 (No Ratings Yet)
(No Ratings Yet)