In Development this week (Vol. 144, Issue 21)
Posted by Seema Grewal, on 31 October 2017
Here are the highlights form the current issue of Development:
Making matrix in the inner ear
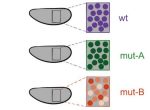
 The tectorial membrane (TM) is an extracellular matrix (ECM) that overlies the organ of Corti in the inner ear and is crucial for our sense of hearing. It is composed of collagen fibrils embedded in a tectorin-based matrix. The precise alignment of the collagen fibrils across the TM is a feature considered critical for hearing, but very little is known about how this pattern is generated. On p. 3978, Richard Goodyear and colleagues undertake a detailed analysis of TM development in mice and begin to investigate the mechanisms underlying collagen-fibril orientation. They find that the presence of a tectorin-based matrix is essential for the normal co-alignment and orientation of the first-forming collagen fibrils, and that collagen-fibril orientation does not seem to depend on stretch of the ECM caused by growth of the underlying epithelium. Rather, the authors identify an influence of the planar cell polarity machinery, generally associated with cell-cell alignment, on collagen fibril orientation – although the molecular mechanisms underlying this remain unclear. These data provide first insights into how TM patterning is achieved, and point to an intriguing interplay between planar cell polarity and collagen-fibril organisation.
The tectorial membrane (TM) is an extracellular matrix (ECM) that overlies the organ of Corti in the inner ear and is crucial for our sense of hearing. It is composed of collagen fibrils embedded in a tectorin-based matrix. The precise alignment of the collagen fibrils across the TM is a feature considered critical for hearing, but very little is known about how this pattern is generated. On p. 3978, Richard Goodyear and colleagues undertake a detailed analysis of TM development in mice and begin to investigate the mechanisms underlying collagen-fibril orientation. They find that the presence of a tectorin-based matrix is essential for the normal co-alignment and orientation of the first-forming collagen fibrils, and that collagen-fibril orientation does not seem to depend on stretch of the ECM caused by growth of the underlying epithelium. Rather, the authors identify an influence of the planar cell polarity machinery, generally associated with cell-cell alignment, on collagen fibril orientation – although the molecular mechanisms underlying this remain unclear. These data provide first insights into how TM patterning is achieved, and point to an intriguing interplay between planar cell polarity and collagen-fibril organisation.
Gastruloids: mimicking early embryonic polarisation in vitro
 Embryonic patterning is dependent on the establishment of the anteroposterior (AP) and dorsoventral axes early in development. In mammalian embryos, these axes are established by a breaking of symmetry in the epiblast, which involves signals from the extra-embryonic tissues. However, the molecular mechanisms that control this process are still not fully understood. On p. 3894, David Turner, Alfonso Martinez Arias and colleagues use gastruloids, three-dimensional aggregates of mouse embryonic stem cells, as a tool to unravel the signalling pathways that establish AP polarity in mammalian embryos. The authors demonstrate that these gastruloids can develop an AP axis in the absence of extra-embryonic tissue, instead depending on precisely timed interactions between Wnt and Nodal signalling. They also show that BMP signalling is dispensable for AP axis formation. This research demonstrates the powerful potential of gastruloids as a tool to understand the molecular mechanisms that underpin early embryonic development. Together, their results suggest that extra-embryonic tissues do not induce axis formation per se, but rather bias the critical symmetry-breaking event in embryo development, furthering our understanding of the molecular control of embryonic patterning.
Embryonic patterning is dependent on the establishment of the anteroposterior (AP) and dorsoventral axes early in development. In mammalian embryos, these axes are established by a breaking of symmetry in the epiblast, which involves signals from the extra-embryonic tissues. However, the molecular mechanisms that control this process are still not fully understood. On p. 3894, David Turner, Alfonso Martinez Arias and colleagues use gastruloids, three-dimensional aggregates of mouse embryonic stem cells, as a tool to unravel the signalling pathways that establish AP polarity in mammalian embryos. The authors demonstrate that these gastruloids can develop an AP axis in the absence of extra-embryonic tissue, instead depending on precisely timed interactions between Wnt and Nodal signalling. They also show that BMP signalling is dispensable for AP axis formation. This research demonstrates the powerful potential of gastruloids as a tool to understand the molecular mechanisms that underpin early embryonic development. Together, their results suggest that extra-embryonic tissues do not induce axis formation per se, but rather bias the critical symmetry-breaking event in embryo development, furthering our understanding of the molecular control of embryonic patterning.
Atg16 in the intestine: more than autophagy
 The core autophagy protein Atg16L1 has been identified as a genetic risk factor in inflammatory bowel disease, but how it plays this role has remained unclear. On p. 3990, Gábor Juhász and colleagues interrogate the role of Atg16, the Drosophila orthologue of human ATG16L1, in intestinal homeostasis and inflammation. Using mutants that affect either the N-terminal autophagic domain or the C-terminal WD40 domain, they observe defects in intestinal morphology and an impaired stress response in Atg16 WD40 mutants. In Atg16 WD40 mutant intestines, the differentiation of enteroendocrine (EE) cells is impaired, leading to an accumulation of pre-EE cells, and this results from reduced Slit/Robo signalling (a pathway known to regulate EE cell number). The failure of EE differentiation is accompanied by an inflammatory response, but appears to be independent of autophagy: autophagy is not altered in Atg16 WD40 mutants, and mutants affecting the autophagy domain alter neither Slit/Robo signalling nor EE differentiation. Finally, the authors show that Atg16 binds to the GTPase Rab19 – also a genetic risk factor for inflammatory bowel disease – and the two cooperate in regulating intestinal homeostasis. This work provides insight into the molecular control of intestinal homeostasis and implies a link between impaired cell differentiation and intestinal pathologies in humans.
The core autophagy protein Atg16L1 has been identified as a genetic risk factor in inflammatory bowel disease, but how it plays this role has remained unclear. On p. 3990, Gábor Juhász and colleagues interrogate the role of Atg16, the Drosophila orthologue of human ATG16L1, in intestinal homeostasis and inflammation. Using mutants that affect either the N-terminal autophagic domain or the C-terminal WD40 domain, they observe defects in intestinal morphology and an impaired stress response in Atg16 WD40 mutants. In Atg16 WD40 mutant intestines, the differentiation of enteroendocrine (EE) cells is impaired, leading to an accumulation of pre-EE cells, and this results from reduced Slit/Robo signalling (a pathway known to regulate EE cell number). The failure of EE differentiation is accompanied by an inflammatory response, but appears to be independent of autophagy: autophagy is not altered in Atg16 WD40 mutants, and mutants affecting the autophagy domain alter neither Slit/Robo signalling nor EE differentiation. Finally, the authors show that Atg16 binds to the GTPase Rab19 – also a genetic risk factor for inflammatory bowel disease – and the two cooperate in regulating intestinal homeostasis. This work provides insight into the molecular control of intestinal homeostasis and implies a link between impaired cell differentiation and intestinal pathologies in humans.
PLUS:
An interview with Christiane Nüsslein-Volhard
 Christiane Nüsslein-Volhard is Director Emeritus at the Max Planck Institute for Developmental Biology in Tübingen, Germany. In 1995, she was awarded the Nobel Prize for Physiology and Medicine, along with Eric Wieschaus and Edward Lewis, for her work on the genetic control of embryogenesis using the fruit fly Drosophila melanogaster. In the 1990s, she transitioned her lab to working with zebrafish (Danio rerio), using similar forward genetic approaches to those that had proved so successful in Drosophila to uncover key regulators of vertebrate development. We met with Christiane at the recent International Society for Developmental Biology (ISDB) meeting in Singapore, to talk about her research, the impact of the Nobel Prize and the challenges of being a ‘woman in science’. See the Spotlight article.
Christiane Nüsslein-Volhard is Director Emeritus at the Max Planck Institute for Developmental Biology in Tübingen, Germany. In 1995, she was awarded the Nobel Prize for Physiology and Medicine, along with Eric Wieschaus and Edward Lewis, for her work on the genetic control of embryogenesis using the fruit fly Drosophila melanogaster. In the 1990s, she transitioned her lab to working with zebrafish (Danio rerio), using similar forward genetic approaches to those that had proved so successful in Drosophila to uncover key regulators of vertebrate development. We met with Christiane at the recent International Society for Developmental Biology (ISDB) meeting in Singapore, to talk about her research, the impact of the Nobel Prize and the challenges of being a ‘woman in science’. See the Spotlight article.
Transcriptional precision and accuracy in development: from measurements to models and mechanisms
 During development, genes are transcribed at specific times, locations and levels. In recent years, the emergence of quantitative tools has significantly advanced our ability to measure transcription with high spatiotemporal resolution in vivo. Here, Angela DePace and co-workers highlight recent studies that have used these tools to characterize transcription during development, and discuss the mechanisms that contribute to the precision and accuracy of the timing, location and level of transcription. See the Review.
During development, genes are transcribed at specific times, locations and levels. In recent years, the emergence of quantitative tools has significantly advanced our ability to measure transcription with high spatiotemporal resolution in vivo. Here, Angela DePace and co-workers highlight recent studies that have used these tools to characterize transcription during development, and discuss the mechanisms that contribute to the precision and accuracy of the timing, location and level of transcription. See the Review.
Cortical interneuron development: a tale of time and space
 Cortical interneurons are a diverse group of neurons that project locally and are crucial for regulating information processing and flow throughout the cortex. Recent studies in mice have advanced our understanding of how these neurons are specified, migrate and mature. Here, John Rubenstein and colleagues evaluate new findings that provide insights into the development of cortical interneurons and that shed light on when their fate is determined, on the influence that regional domains have on their development, and on the role that key transcription factors and other crucial regulatory genes play in these events. See the Review.
Cortical interneurons are a diverse group of neurons that project locally and are crucial for regulating information processing and flow throughout the cortex. Recent studies in mice have advanced our understanding of how these neurons are specified, migrate and mature. Here, John Rubenstein and colleagues evaluate new findings that provide insights into the development of cortical interneurons and that shed light on when their fate is determined, on the influence that regional domains have on their development, and on the role that key transcription factors and other crucial regulatory genes play in these events. See the Review.


 (No Ratings Yet)
(No Ratings Yet)