From Tip to Grain: Sculpting Barley Inflorescence Through the Regulation of Meristem Activity
Posted by Isaia Vardanega, on 12 June 2025
Written by Isaia Vardanega
Behind the Paper Story of “CLAVATA signalling shapes barley inflorescence by controlling activity and determinacy of shoot meristem and rachilla”.
Grasses play a crucial role in human food production and are primarily cultivated for their edible grains. The quantity of grain a grass species can produce is closely linked to the architecture of its inflorescence. Over the course of evolution, grasses have developed a wide variety of inflorescence architectures. From the complex branched inflorescences of the Oryzae tribe (e.g. rice), where multiple grains develop on primary and secondary branches, to the simple spike-type inflorescence of the Triticeae tribe (e.g barley and wheat), where single grains develop on short vestigial axes called rachillae1 (Fig.1 A).
These architectural differences are established during the early stages of the plant’s development by the activity of meristems, specialised structures leading organ growth. The size, position, and lifespan of these meristems determine the eventual shape of the inflorescence2. An example of how small differences in meristem activity can significantly impact the final inflorescence architecture is found within the Triticeae tribe. In barley, the rachilla primordium grows just enough to form a single floret and grain. In contrast, in wheat, its prolonged activity allows for the formation of multiple florets per spikelet, ultimately resulting in its characteristic multi-grain spikelet1 (Fig.1 B,C).
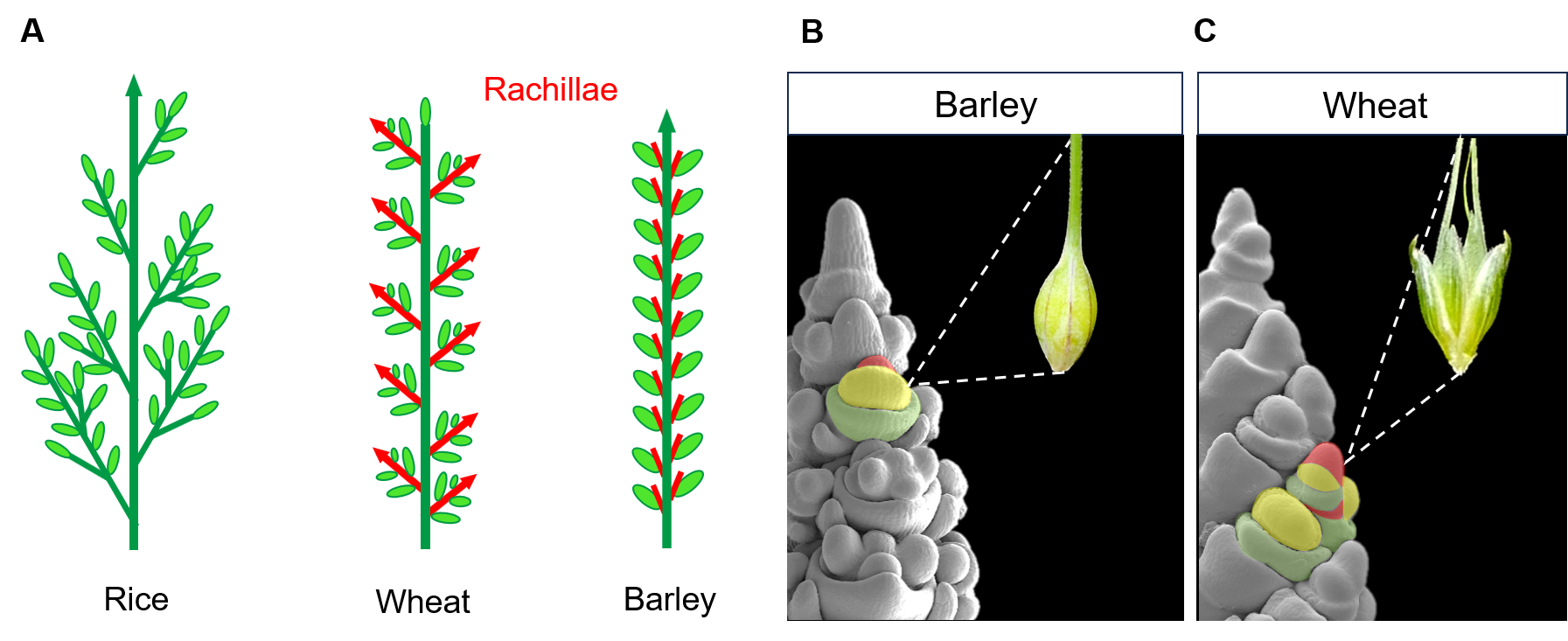
Figure 1: (A) Schematic representation of different grass inflorescence architectures. Main stem and branches in dark green, grains in light green and rachillae in red. (B,C) Scanning electron microscope images of barley and wheat inflorescences at early stages of development. Different organs comprising a spikelet are coloured (rachilla primordium in red, floret meristem in yellow and lemma primordium in green). Each spikelet develops into a single grain in barley and multiple grains in wheat.
When I joined Rüdiger Simon’s lab to begin my PhD, the group was primarily focused on understanding the role of CLE signalling pathways in regulating shape, size, and maintenance of shoot and root apical meristems in Arabidopsis thaliana. At that time, they had begun extending their research to the cereal plant barley. Gwendolyn Kirschner, a former PhD student in the lab, had started investigating the role of CLE-peptide signalling in barley by generating fluorescent reporter lines, including the barley orthologs of the Arabidopsis CLE40 peptide (HvFCP1) and CLV1 receptor (HvCLV1), which had previously been shown to regulate stem cell fate in Arabidopsis meristems3,4. While Gwendolyn primarily analysed barley roots, my project focused more on shoot apical meristem and inflorescence development.
In comparison to the simple inflorescence of Arabidopsis, grasses evolved a more complex organisation, with different meristem types leading to the formation of various organs comprising the spikelet, the basic unit responsible for the development of grains in cereals. This observation led us to the questions: “How is the shape and activity of all these different meristems regulated and coordinated to generate specific inflorescences in grasses? Did barley evolve specific CLE/CLAVATA signalling pathways to regulate the activity of different meristem types?”
HvCLV1 regulates meristem activity along the vertical and lateral axes of the barley inflorescence.
I began by mutating the closest ortholog of CLV1 in barley (HvCLV1) as well as other closely related receptors, which we are still investigating. In maize, as in Arabidopsis, mutation of CLV1 or its ortholog leads to a drastically enlarged inflorescence meristem, resulting in the disorganised formation of additional primordia4,5. In the barley Hvclv1 mutant, I initially observed occasional formation of extra grains within the inflorescence. However, detailed analysis by scanning electron microscopy revealed that an enlarged inflorescence meristem generated an additional row of spikelet primordia, organised in a spiral phyllotaxis rather than in a disorganised manner (Fig. 2A, B).
Moreover, I noticed that Hvclv1 inflorescences developed an elongated rachilla primordium, which produced additional florets per spikelet, an effect previously observed in barley mutants as multiflorus2.b and INTERMEDIUM-m6,7. These results led me to conclude that the ectopic grains generated by the Hvclv1 mutant were due to increased activity of the inflorescence meristem along the vertical axis and of the rachilla primordium along the lateral axis. To further support this, I imaged mature rachillae from WT and Hvclv1 plants and observed the formation of a meristem-like structure at the tip of the Hvclv1 rachilla, which developed into an actively growing small branch instead of the vestigial hairy structure seen in WT (Fig. 2C, D).
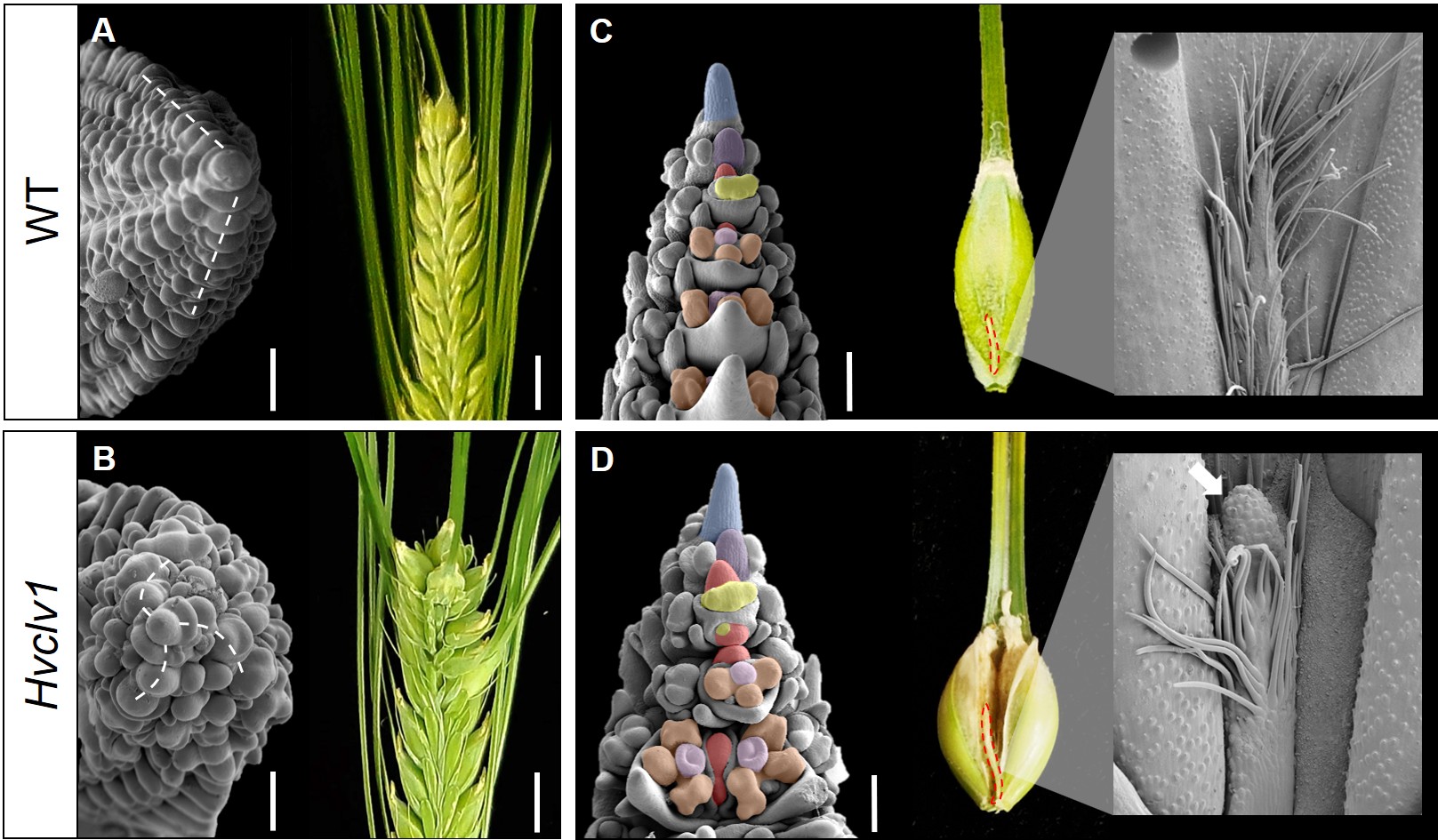
Figure 2: (A,B) Scanning electron microscope images displaying spikelet phyllotaxis (dashed lines) in WT (A) and Hvclv1 (B) early inflorescence tips, combined with photos of the respective final inflorescences. (C,D) Scanning electron microscope images of WT and Hvclv1 early inflorescences. Colours were used to highlight different meristems and primordia (inflorescence meristem in blue, spikelet meristem in violet, rachilla primordium in red, floret meristem in yellow, anther and carpel primordia in brown and pink). Red dashed lines indicate the mature rachilla, combined with zoom-in images where the white arrow indicates the meristem-like structure identified in Hvclv1. Figure modified from Vardanega et. al 2025.
The CLE peptide HvFCP1 acts with HvCLV1 to restrict rachilla primordium activity to the formation of a single floret.
Once the function of the HvCLV1 receptor was characterised, I wondered whether the regulation of rachilla growth was determined by the binding of a specific CLE peptide. Barley possesses 28 different CLE peptides, but I was specifically seeking one that is strongly conserved among grasses and may have contributed to the drastic reduction of branch size in Triticeae. Upon reviewing the literature, I realised that only one peptide retained the same protein sequence across all studied grass species: FCP18. HvFCP1 is the closest ortholog to the Arabidopsis CLE40, for which we already had a fluorescent reporter line. When I examined the expression of HvFCP1 during barley inflorescence development, I noticed that it was not only co-expressed with HvCLV1 but also specifically expressed in the rachilla primordium (Fig. 3 A,B). The insensitivity of the Hvclv1 shoot apical meristem to HvFCP1 peptide treatment, along with the formation of an elongated rachilla producing additional florets even in Hvfcp1 mutants (Fig. 3 C), ultimately demonstrated that HvFCP1 interacts with HvCLV1 to regulate rachilla activity in barley, thereby determining its specific inflorescence architecture.
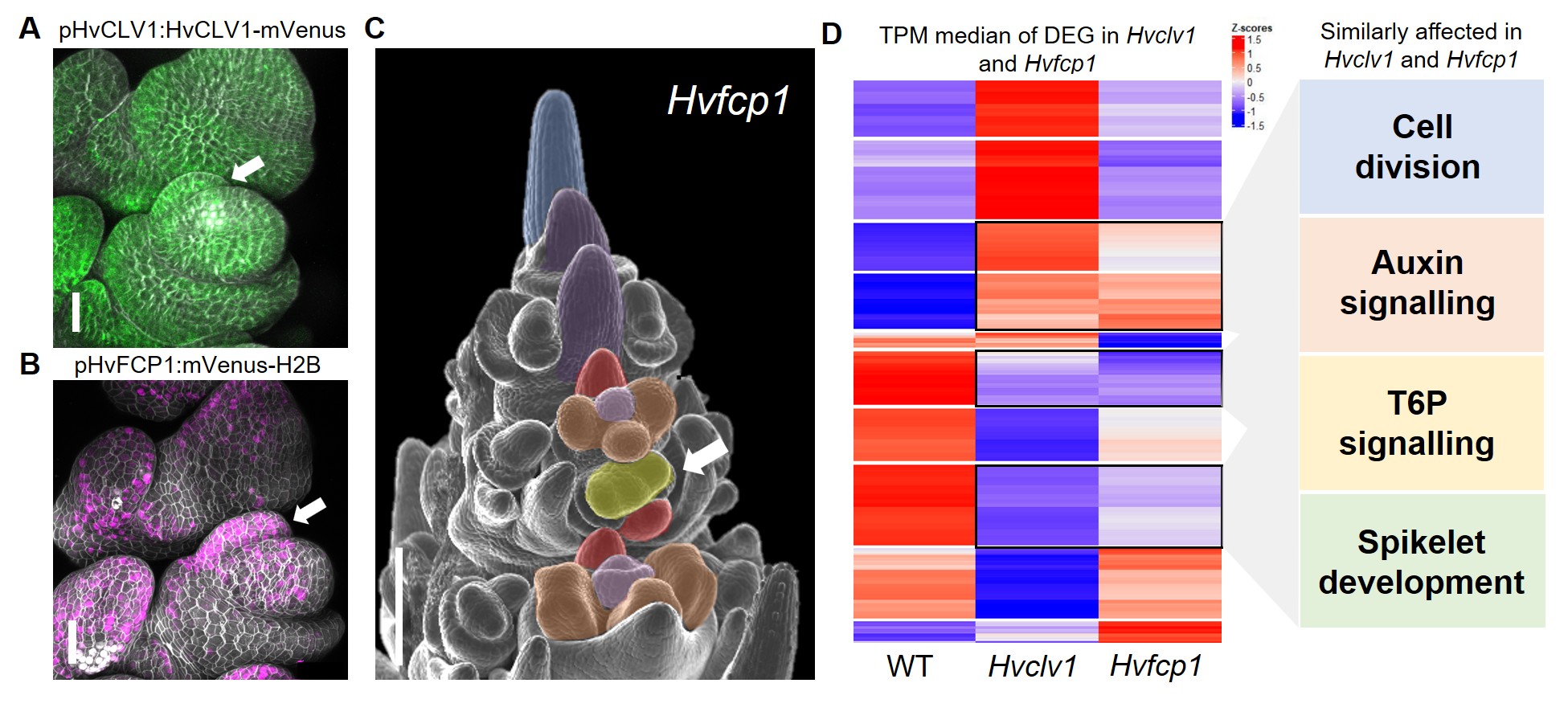
Figure 3: (A,B) Confocal microscope pictures of barley spikelets displaying HvCLV1 protein localisation (green) and HvFCP1 promoter activity (magenta). The white arrow indicates the rachilla primordium. (C) Hvfcp1 inflorescence displaying double florets (white arrow). (D) RNA seq results. The heatmap shows the z-score of median Transcripts Per Million (TPM) values for each of the Differently Expressed Genes (DEG) in Hvclv1 vs WT and Hvfcp1 vs WT. Black rectangles indicate similarly differentially expressed genes between Hvclv1 vs WT and Hvfcp1 vs WT, with the affected biological processes on the side. Figure modified from Vardanega et. al 2025.
I then investigated which genes are directly or indirectly regulated by HvFCP1/HvCLV1 by performing RNA sequencing on mutant inflorescences compared to wild type (WT). The transcriptome analysis revealed several similarly differentially expressed genes (DEG) between Hvclv1 vs WT and Hvfcp1 vs WT, involved in processes such as cell division, auxin signalling, and trehalose-6-phosphate signalling, providing possible target genes that we are currently investigating (Fig.3 D).
Translating knowledge and techniques from model plants to crops.
In addition to its biological novelty, this paper represents a successful example of translational research, bridging techniques and knowledge from model species to agronomically significant crops. We applied various microscopy techniques more commonly used in model plants, such as Arabidopsis thaliana, but less frequently employed in cereal biology. We developed reporter and complementation lines and quantified receptor cytoplasmic internalisation in rachilla and floret meristems. Furthermore, we utilised methods such as 3D reconstruction and smRNA-FISH for detailed phenotypic analysis of the inflorescence meristem and its expression patterns.
To build on this approach, we generated BARVISTA (http://purl.org/barvista/home), a dataset providing transcriptional information for each cell within the barley inflorescence by integrating single-cell RNA sequencing data with spatial transcriptomics results. Using this resource, we identified transcription factors involved in establishing the specific patterns of meristem ontogenesis necessary to shape the characteristic morphology of the barley spike9.
In conclusion, our work shed light on the signalling pathways that regulate the shape and behaviour of individual meristem types within the inflorescence, paving the way for future efforts to engineer inflorescence architecture through targeted regulation of distinct meristem activities.
REFERENCES:
- Koppolu, R. & Schnurbusch, T. Developmental pathways for shaping spike inflorescence architecture in barley and wheat. Journal of Integrative Plant Biology 61, 278–295 (2019).
- Kyozuka, J., Tokunaga, H. & Yoshida, A. Control of grass inflorescence form by the fine-tuning of meristem phase change. Current Opinion in Plant Biology 17, 110–115 (2014).
- Berckmans, B., Kirschner, G., Gerlitz, N., Stadler, R. & Simon, R. CLE40 Signaling Regulates Root Stem Cell Fate. Plant Physiol 182, 1776–1792 (2020).
- Schlegel, J. et al. Control of Arabidopsis shoot stem cell homeostasis by two antagonistic CLE peptide signalling pathways. eLife 10, e70934 (2021).
- Bommert, P. et al. thick tassel dwarf1 encodes a putative maize ortholog of the Arabidopsis CLAVATA1 leucine-rich repeat receptor-like kinase. Development 132, 1235–1245 (2005).
- Koppolu, R. et al. The barley mutant multiflorus2.b reveals quantitative genetic variation for new spikelet architecture. Theor Appl Genet 135, 571–590 (2022).
- Zhong, J. et al. INTERMEDIUM-M encodes an HvAP2L-H5 ortholog and is required for inflorescence indeterminacy and spikelet determinacy in barley. Proceedings of the National Academy of Sciences 118, e2011779118 (2021).
- Goad, D. M., Zhu, C. & Kellogg, E. A. Comprehensive identification and clustering of CLV3/ESR-related (CLE) genes in plants finds groups with potentially shared function. New Phytologist 216, 605–616 (2017).
- Demesa-Arevalo, E. et al. Imputation integrates single-cell and spatial gene expression data to resolve transcriptional networks in barley shoot meristem development. 2025.05.09.653223 Preprint at Biorxiv https://doi.org/10.1101/2025.05.09.653223 (2025).


 (No Ratings Yet)
(No Ratings Yet)