How exploring single cell dynamics in the early embryo revealed a protective system based on mechanical cell cooperation
Posted by Esteban Hoijman, on 15 July 2021
by Esteban Hoijman and Verena Ruprecht
The immune system is able to deal with cellular anomalies and invading pathogens. However, it has remained enigmatic how vertebrate embryos handle stress conditions before such an immune system develops. We recently found that embryos are able to perform innate immune functions as early as in blastula stage of development, using the surface epithelium as a phagocytic clearance system of defective cells1. This epithelium, the trophoblast in mammalian embryos, is the first differentiated tissue that forms during embryonic development, and our study revealed that it has unrecognized important functions for the survival of the early embryo.
The story began when Esteban joined as a postdoctoral researcher the recently established lab of Verena at the Centre for Genomic Regulation in Barcelona, Spain. With the aim to study early vertebrate embryo development under native and stress conditions we started to acquire movies of single cell dynamics at high spatiotemporal resolution in the zebrafish blastula. Interestingly, we noticed that epithelial cells of the embryo surface (termed enveloping layer in zebrafish) were able to ingest fragments of internal stem cells, forming vacuolar like structures in epithelial cells (Figure 1). We further observed that epithelial uptake was specifically increased in stressed embryos that showed signs of malformations or tissue damage caused by mechanical perturbation, suggesting that the ingested cells originated from cellular failures or events of cell death. In fact, we were able to confirm that the surface epithelium specifically ingested dead cells, as the ones generated in normal embryo development by errors in mitosis. This led us to think that the epithelial tissue at the embryo surface formed during early development (only four hours post fertilization in zebrafish embryos), could function as a scavenger of dead cells at these stages. This was a challenging idea, as specific clearance functions of epithelial cells have not been previously considered to occur in the early embryo. Phagocytosis of dead cells by epithelia was known to be important in certain adult tissues2-3, suggesting that epithelial cells could perform similar functions in the early embryo. We reasoned that this function could have an essential protective role in development, as an accumulation of dead bodies might lead to multiple defects, from mechanical interference with gastrulation movements to the uncontrolled release of intracellular contents by secondary necrosis, including signaling molecules and toxic factors.
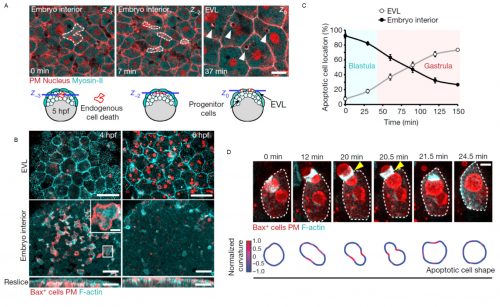
Motivated by the possible relevance of these observations, Esteban combined his expertise in live embryo imaging with experience in cell apoptosis that he gained during his PhD. We developed a single cell in vivo morphodynamic analysis of phagocytosis that allowed us to study epithelial phagocytic events inside a living animal (Figure 1B). Our study revealed that the phagocytic cup formed by epithelial cells exerts compressive forces during uptake of an apoptotic cell, a feature recently discovered to be shared by macrophages ingesting synthetic phagocytic targets in culture4.
Next, we decided to evaluate if this process was conserved in mammals, as this could have relevant implications for the progression of human embryonic development. Filming mouse blastocysts, we observed that the surface epithelium (in this case the trophoblast) was also able to clear apoptotic cells by recognizing surface molecules of dying cells, specifically phosphatidylserine. This observation indicated that the early epithelial phagocytic process we first observed in zebrafish embryos was evolutionary conserved and based on similar molecular mechanisms to the ones used by professional phagocytic cells of the immune system such as macrophages. These results were exciting, as they suggested that the first tissue formed at the surface of a vertebrate embryo is used for its protection. This is particularly relevant considering that the main cause of early miscarriages in human embryos are errors in mitosis leading to cell death5.
We observed that phagocytic clearance was sensitive enough to detect single cells dying, while being efficient enough to be able to clear hundreds of cells dying simultaneously (Figure 1). We were thus wondering how epithelial cells, which are sessile cells in the epithelium, can achieve such high efficiency for clearance, as compared to immune phagocytes which are motile to perform their (clearance) task. The answer was hidden in our movies of live zebrafish embryos. We detected two surprising features: first, dead cells moved much more than what we expected before the uptake, significantly faster than the live neighboring cells undergoing gastrulation movements. Although it was proposed that apoptotic cells can acquire specific motility inside tissues, this was not directly proven to be cell-autonomous6-8. Our dynamic analysis of actomyosin network organization, which showed a static localization and non-polarized organization in apoptotic cells, was however supporting that they do not have autonomous motility. Second, by visualizing F-actin dynamics inside the whole embryo, we observed the formation of actin accumulations in contact with the external rear surface of motile apoptotic cells. We therefore questioned, could these two observations be linked? Surprised by the observation of these actin structures, we performed both high-speed imaging to analyze actin localization dynamics and single cell staining to identify the cells in which they are formed. This allowed us to determine that these actin structures formed in epithelial cells and constitute a new elongated protrusion, with actin enriched at the protrusion tip in contact with apoptotic cells and moving coordinately with them, a protrusion type that we called “epithelial arms” (Figure 2A,B).
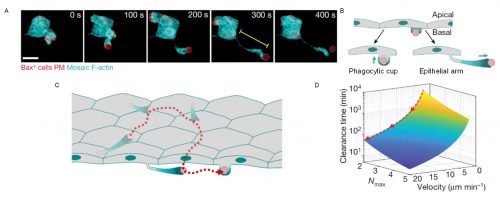
Figure 2. Epithelial arms propel apoptotic cells improving tissue clearance efficiency. (A) An epithelial arm forms upon contacting an apoptotic cell (red). (B) Epithelial cells form two types of protrusions when recognize an apoptotic cell: phagocytic cups to ingest and epithelial arms to push. (C) Consecutive pushing by epithelial arms increases the number of epithelial cells encountered by the each apoptotic cell (red). (D) Modeling indicates that the time to complete clearance of an apoptotic mass is reduced when velocity of the apoptotic targets increases. Nmax: maximum number of targets ingested by each epithelial cell. Adapted from Hoijman et al, Nature 2021, 590:618.
We came up with different hypotheses that might explain this observation: Could these protrusions mechanically push the apoptotic cells? Or are protrusions just “chasing” apoptotic cells? In the latter case, we should probably observe some apoptotic cells moving without contacting epithelial arms. However, we observed a stringent correlation between arms and apoptotic movement with both occurring hand in hand. To evaluate our first hypothesis, we designed an experiment in which we transplanted synthetic apoptotic targets made of lipid aggregates into the embryo as a source of objects with no intrinsic motility. Interestingly, these targets were efficiently phagocytosed and, furthermore, they acquired similar motility as apoptotic cells in terms of velocity fluctuations, directionality, and association with epithelial arms. The movement of the surrogate targets indicated that epithelial arms can propel apoptotic targets using actin-dependent forces. Looking at the whole tissue, we further found that apoptotic cells moved along long-range trajectories, caused via consecutive pushing by multiple epithelial arms formed by different epithelial cells (Figure 2C), with the path for each dead cell target being described by a random-walk.
We therefore asked, how does this apoptotic target movement influence the overall efficiency of tissue clearance? In other words, from the point of view of a single epithelial cell, why would it be meaningful to push a target instead of ingesting it directly? Analyzing population movement of apoptotic targets, we observed that the random-walk behavior led to the overall spreading of the apoptotic mass. A larger area of the epithelial tissue was therefore “visited” by apoptotic targets over time. Comparing this behavior to professional phagocytes, which usually move towards an apoptotic cell mass to perform efficient clearance, we interpreted the observed spreading of the apoptotic mass as an inversion of the clearance process, with motile targets moving around sessile epithelial phagocytes.
The immediate consequence of apoptotic motility was an increased number of epithelial cells encountered by each target. We hypothesized that several possibilities could lead to an advantage for cells clearing the tissue when increasing the phagocyte-target encounter rate. We therefore set out to mathematically model the clearance process and its efficiency. For this, Verena teamed up with Stefan Wieser (ICFO Barcelona) and Andrew Callan-Jones (CNRS/Université de Paris) to perform simulations and derived a theoretical formula that predicted that increasing the speed of target spreading (mediated by arm pushing) decreases the time required to clear the whole apoptotic mass, thus making the process more efficient (Figure 2D). Importantly, this was specifically relevant when a limited uptake capacity for each epithelial cell exists, as we determined experimentally.
The successful combination of this theoretical and experimental analysis indicated that the stochastic spreading allows the recruitment of new epithelial phagocytes near the vicinity of those cells that already reached their maximal uptake capacity, thereby accelerating the clearance process at the tissue level.
In summary, the mechanical cooperation between epithelial cells mediated by the active dispersal of apoptotic targets by epithelial arms improves the protection of the embryo at the earliest stages of development. We are now exploring the impact of this phagocytic system at the CRG Barcelona (Verena) and the newly established group at the University of Barcelona (Esteban).
As many other researchers experienced in 2020-2021, performing a revision in the peak of the pandemic was a challenging situation that led us to deal with complex situations and demanded creative solutions and support between labs to solve unpredictable problems (i.e. we needed a reagent during the revision when companies paused their supply; we were lucky to receive it as a gift from the lab next door). We believe that our work is a good example of how advanced imaging technologies that can nowadays capture single cell and molecular dynamics inside live animals open new exciting opportunities for the discovery of relevant in vivo cell and tissue functions and their underlying mechanisms.
- Hoijman et al, Nature 2021, 590:618, doi: 10.1038/s41586-021-03200-3
- Akhtar et al, Dev Cell 2016, 38:522, doi: 10.1016/j.devcel.2016.08.005
- Juncadella et al, Nature 2013, 493:547, doi: 10.1038/nature11714
- Vorselen et al, bioarxiv 2021, doi.org/10.1101/2021.03.14.435346
- Bolton et al, Nat Commun 2016, 7:11165, doi: 10.1038/ncomms11165
- Yamaguchi et al, J Cell Biol 2011, 195:1047, doi: 10.1083/jcb.201104057
- van Ham, Curr Biol 2012, 22:830, doi: 10.1016/j.cub.2012.03.027
- Dzhagalov et al, PLoS Biol 2013, 11:e1001566, doi: 10.1371/journal.pbio.1001566


 (1 votes)
(1 votes)