In Development this week (Vol. 139, Issue 5)
Posted by Seema Grewal, on 8 February 2012
Here are the highlights from the current issue of Development:
ROCKed to the heart
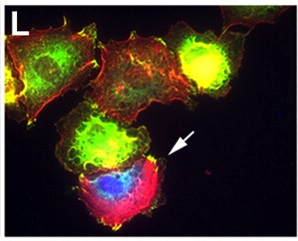
Noonan syndrome – a common cause of congenital heart disease – is often associated with missense mutations in the protein phosphatase SHP-2. Interestingly, some types of leukaemia are associated with another subset of SHP-2 missense mutations. Here (p. 948), Frank Conlon and colleagues introduce SHP-2 that contains Noonan-associated mutations or juvenile myelomonocytic leukaemia (JMML)-associated mutations into Xenopus embryos to investigate how SHP-2 regulates heart development. Embryos that express SHP-2 containing Noonan-associated mutations have morphologically abnormal hearts, they report, whereas embryos that express SHP-2 containing JMML-associated mutations have normal hearts. The cardiac abnormalities caused by the Noonan-associated mutations are coupled with a delay or arrest in the cardiac cell cycle and with defective incorporation of cardiomyocyte precursors into the developing heart. Notably, these defects, which are caused by disruptions in the polarity of cardiac actin fibres and in F-actin deposition, can be rescued by inhibition of the Rho-associated, coiled-coil-containing protein kinase 1 (ROCK), which indicates that SHP-2 acts via ROCK to regulate the cardiac actin cytoskeleton during heart development.
Breaking symmetry during lateral root formation
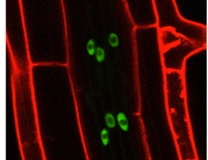
Lateral root (LR) formation, which is essential for the construction of plant root systems, is initiated by coordinated asymmetric cell divisions (ACDs) of LR founder cells in existing roots. In Arabidopsis, LR formation is regulated by auxin signalling through the auxin response factors ARF7 and ARF19, which transcriptionally activate the plant-specific transcriptional regulator LBD16/ASL18 and other LBD/ASL genes. Hidehiro Fukaki and colleagues now provide new insights into the biological role of LBD/ASL genes in LR formation (see p. 883). They show that LBD16/ASL18 is expressed in Arabidopsis LR founder cells prior to ACD and that the spatiotemporal expression of LBD16/ASL18 during LR initiation is dependent on a specific auxin signalling module. Moreover, inhibition of LBD16/ASL18 and related LBD/ASL proteins in LR founder cells blocks nuclear migration, ACD and LR initiation. These results indicate that the localised activity of LBD16/ASL18 and related LBD/ASL proteins establishes the asymmetry of LR founder cells that is required for LR initiation, a key step in the construction of the plant root system.
Untangling the Hairy segmentation clock
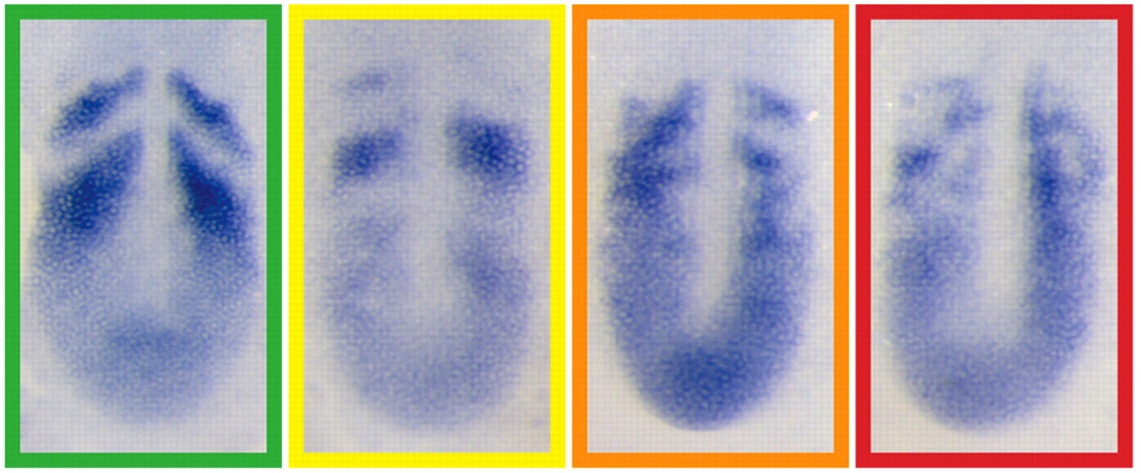
During somitogenesis, an oscillating gene network generates a segmental pattern in the presomitic mesoderm. In zebrafish, this segmentation clock centres on cycles of transcription and self-repression of numerous hairy-enhancer of split related (her) genes, which encode proteins that dimerise, bind DNA and repress transcription. On p. 940, Scott Holley and co-workers systematically examine the physical interactions between Her proteins and test the ability of various Her protein dimers to bind to cis regulatory sequences. Dimerisation of Her proteins is specific, they report, with Hes6 serving as the hub of the network. Not all dimers bind to DNA, they note, but those that do so have distinct preferences for different cis regulatory sequences. Finally, Her7 disproportionately influences the availability of Hes6 for heterodimerisation with other Her proteins. The researchers propose, therefore, that Her7 has two functions within the zebrafish segmentation clock – direct repression of transcription through formation of a DNA-binding heterodimer with Hes6 and modulation of the network topology via sequestration of the network hub.
Diet and stem cells TORtuously linked
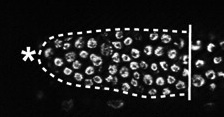
Nutritional status must be coupled to stem/progenitor cell proliferation and differentiation to ensure the proper growth and homeostasis of tissues, but how does diet regulate stem cell behaviour? On p. 859, E. Jane Albert Hubbard and colleagues show that rsks-1 [the homologue of mammalian p70 ribosomal S6 kinase (S6K), a target of the serine/threonine kinase TOR, which regulates cell growth and proliferation in response to nutritional cues] links cell fate, cell cycle and nutrient response in C. elegans germline stem/progenitor cells. They show that rsks-1 is required germline-autonomously to establish the proper number of germline progenitors, a role that requires a conserved TOR phosphorylation site in RSKS-1. Their analysis also reveals genetic interactions between rsks-1 and Notch, which suggest a prominent role for rsks-1 in cell fate control. Furthermore, dietary restriction causes germline defects similar to those observed in rsks-1 mutants and loss of rsks-1 renders the germline largely insensitive to dietary restriction. The researchers propose, therefore, that TOR-S6K signalling is a key nutrient-responsive regulator of germline progenitors.
Apical ECM and epithelial junction integrity
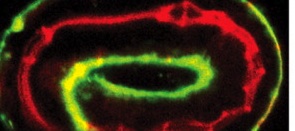
Polarised epithelial cells form many of the body’s surfaces, including the outer epidermis and the lining of several internal tubular organs. The apical surfaces of these epithelial sheets secrete a specialised extracellular matrix (ECM) that is generally viewed as a passive protective layer against pathogens, but does apical ECM have any other roles? According to Meera Sundaram and colleagues, the apical ECM in C. elegans might help to maintain epithelial junction integrity and, consequently, epithelial tissue integrity (see p. 979). The researchers report that the extracellular leucine-rich repeat only (eLRRon) proteins LET-4 and EGG-6 are expressed on the apical surfaces of epidermal cells and some tubular epithelia, including those of the worm’s excretory system. Mutants lacking one or more of these proteins, they report, have multiple defects in apical ECM organisation and, although epithelial junctions initially form correctly in these mutants, they subsequently rupture. Together, these results suggest that eLRRon-dependent apical ECM organisation might modulate epithelial junction dynamics and integrity.
Bimodal control of HoxD gene transcription
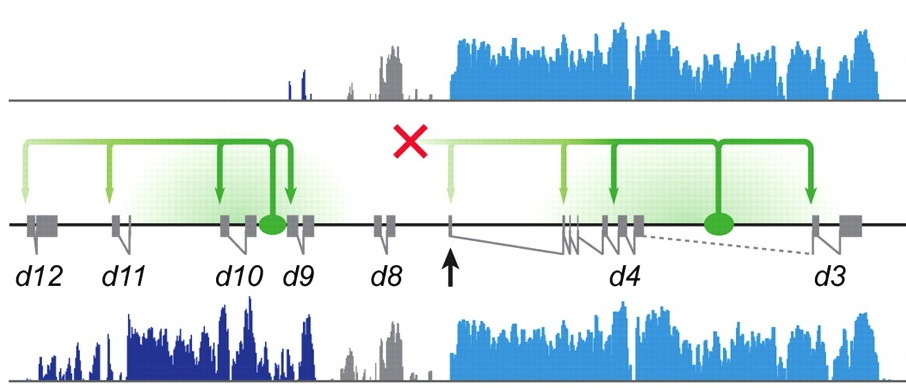
Correct innervation of peripheral muscles by spinal cord motoneurons is required to coordinate body movements in vertebrates. Hox proteins play an important functional role in achieving this innervation by specifying neuronal fates along the anteroposterior axis of the developing spinal cord. However, the mechanisms that generate Hox gene expression patterns are poorly understood. Here (p. 929), Denis Duboule and colleagues use tiling array-based transcriptome analyses and targeted deletions in vivo to investigate the control of HoxD gene transcription in the developing mouse spinal cord. They report that there are two distinct blocks of HoxD transcription that are regulated independently and that define two general expression territories. These territories, they show, are associated with the future nerve plexii at the brachial and lumbar levels. Given these and other results, the researchers propose that the establishment of spatial collinear HoxD domains in the developing mouse spinal cord involves the bimodal control of HoxD gene transcription by two independent ‘enhancer mini-hubs’.
Plus…
Human pre-implantation embryo development
Renee Reijo Pera and colleagues summarize what is currently known about human pre-implantation embryo development and highlight how further studies of human pre-implantation embryos can be used to improve ART and to fully harness the potential of hESCs for therapeutic goals. See the Primer article on p. 829
Retinoic acid signalling during development
Retinoic acid is a vitamin A-derived signaling molecule that acts as ligand for nuclear receptors, converting them from transcriptional repressors to activators. Here, Muriel Rhinn and Pascal Dolle review the main functions of retinoic acid during embryogenesis. See the Primer article on p. 843


 (No Ratings Yet)
(No Ratings Yet)