In Development this week (Vol. 140, Issue 14)
Posted by Seema Grewal, on 3 July 2013
Here are the highlights from the latest issue of Development.
Extrinsic cue for dendrite polarisation
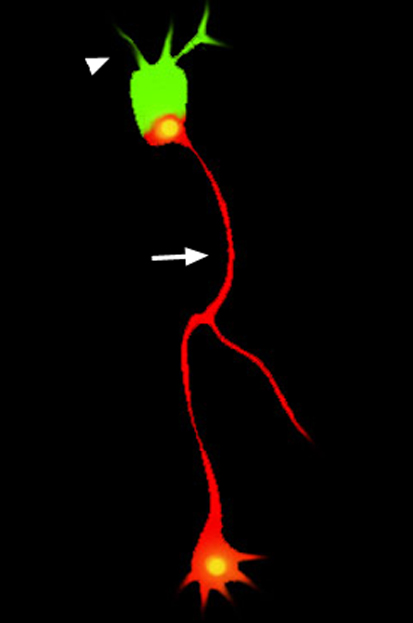 Most neurons have a single axon on one side of their cell body and multiple dendrites on the opposite side. The establishment of this polarisation, which is essential for neuronal function, probably involves both intrinsic and extrinsic factors. Although several intrinsic factors have been identified, the identity of the in vivo extrinsic signals remains unclear. To remedy this situation, Sarah McFarlane and co-workers (p. 2933) have been studying dendrite polarisation in Xenopus retinal ganglion cells (RGCs). They report that neuropilin-1 and plexinA1, which form a holoreceptor for members of the axon guidance family of class III secreted semaphorins (Sema3s), are necessary to bias dendrite extension to the apical side of RGCs in vivo. They report that sema3a and sema3f are expressed on the basal and apical sides of the Xenopus RGC, respectively. Moreover, ectopically expressed Sema3s and inhibition of receptor signalling disrupt dendrite polarisation. The researchers suggest that neuropilin-1 and plexinA1 are co-receptors for an extrinsic cue, probably a Sema3, that directs RGC dendrite polarisation independent of axon polarisation.
Most neurons have a single axon on one side of their cell body and multiple dendrites on the opposite side. The establishment of this polarisation, which is essential for neuronal function, probably involves both intrinsic and extrinsic factors. Although several intrinsic factors have been identified, the identity of the in vivo extrinsic signals remains unclear. To remedy this situation, Sarah McFarlane and co-workers (p. 2933) have been studying dendrite polarisation in Xenopus retinal ganglion cells (RGCs). They report that neuropilin-1 and plexinA1, which form a holoreceptor for members of the axon guidance family of class III secreted semaphorins (Sema3s), are necessary to bias dendrite extension to the apical side of RGCs in vivo. They report that sema3a and sema3f are expressed on the basal and apical sides of the Xenopus RGC, respectively. Moreover, ectopically expressed Sema3s and inhibition of receptor signalling disrupt dendrite polarisation. The researchers suggest that neuropilin-1 and plexinA1 are co-receptors for an extrinsic cue, probably a Sema3, that directs RGC dendrite polarisation independent of axon polarisation.
Bi-polarity in tubulogenesis
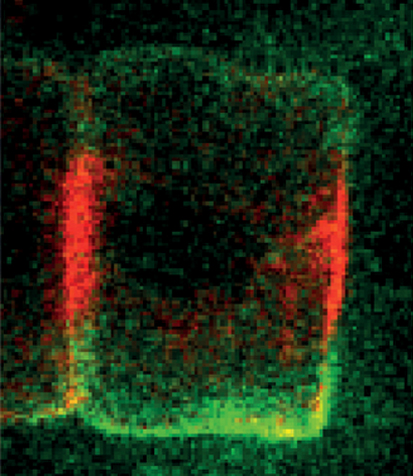 Apico-basal polarisation is a crucial step in the formation of biological tubes. In model systems in which tubulogenesis occurs in cell clusters, the inner surface of each cell in the cluster differentiates into an apical domain where lumen secretion occurs, thus ensuring the formation of an unobstructed lumen. But in many developmental contexts, tubes form from simple cords of cells, which presents a unique challenge for the formation of a continuous lumen. On p. 2985, Di Jiang and colleagues investigate how this challenge is overcome during tubulogenesis in the Ciona intestinalis notochord, which is made up of a single file of geometrically identical cells. The researchers show that, during early tubulogenesis, a patch that contains the highly conserved Par complex and a set of tight junctions becomes established at both ends of the notochord cells. The formation of these two apical domains, they report, is controlled by Par3. Together, these results suggest a new mechanism for tubulogenesis from a simple cell cord that requires the formation of bi-apical cells.
Apico-basal polarisation is a crucial step in the formation of biological tubes. In model systems in which tubulogenesis occurs in cell clusters, the inner surface of each cell in the cluster differentiates into an apical domain where lumen secretion occurs, thus ensuring the formation of an unobstructed lumen. But in many developmental contexts, tubes form from simple cords of cells, which presents a unique challenge for the formation of a continuous lumen. On p. 2985, Di Jiang and colleagues investigate how this challenge is overcome during tubulogenesis in the Ciona intestinalis notochord, which is made up of a single file of geometrically identical cells. The researchers show that, during early tubulogenesis, a patch that contains the highly conserved Par complex and a set of tight junctions becomes established at both ends of the notochord cells. The formation of these two apical domains, they report, is controlled by Par3. Together, these results suggest a new mechanism for tubulogenesis from a simple cell cord that requires the formation of bi-apical cells.
Plants and animals converge to imprint
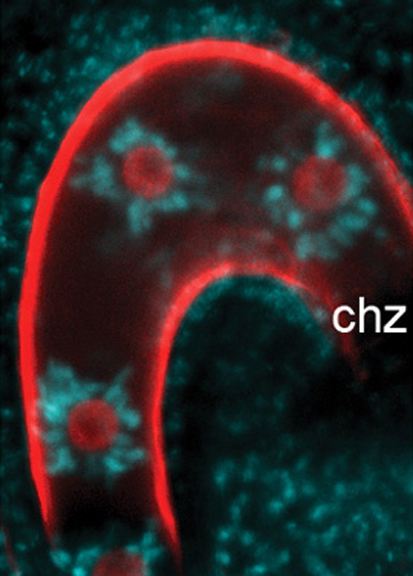 In mammals and plants, parental genomic imprinting, which results from mitotically stable epigenetic modifications, restricts the expression of specific loci to one parental allele. During gametogenesis in mammals, imprinting involves sex-dependent de novo DNA methylation and non-coding RNAs but does a comparable mechanism operate in plants? Here (p. 2953), Thiet Minh Vu, Frédéric Berger and colleagues report that de novo RNA-directed DNA methylation (RdDM), which depends on small interfering RNAs, regulates imprinting at several loci in Arabidopsis endosperm. By dissecting the expression of various members of the RdDM pathway, the researchers show that RdDM is required in somatic tissues to silence both parental alleles, whereas repression of RdDM in female gametes contributes to the activation of the maternal allele. Hence, both de novo DNA methylation and non-coding RNAs play a role in the regulation of imprinted loci in plants and mammals, which suggests that convergent evolutionary processes contribute to imprinting in these distinct groups of eukaryotes.
In mammals and plants, parental genomic imprinting, which results from mitotically stable epigenetic modifications, restricts the expression of specific loci to one parental allele. During gametogenesis in mammals, imprinting involves sex-dependent de novo DNA methylation and non-coding RNAs but does a comparable mechanism operate in plants? Here (p. 2953), Thiet Minh Vu, Frédéric Berger and colleagues report that de novo RNA-directed DNA methylation (RdDM), which depends on small interfering RNAs, regulates imprinting at several loci in Arabidopsis endosperm. By dissecting the expression of various members of the RdDM pathway, the researchers show that RdDM is required in somatic tissues to silence both parental alleles, whereas repression of RdDM in female gametes contributes to the activation of the maternal allele. Hence, both de novo DNA methylation and non-coding RNAs play a role in the regulation of imprinted loci in plants and mammals, which suggests that convergent evolutionary processes contribute to imprinting in these distinct groups of eukaryotes.
Rubbing out epigenetic marks in PGCs
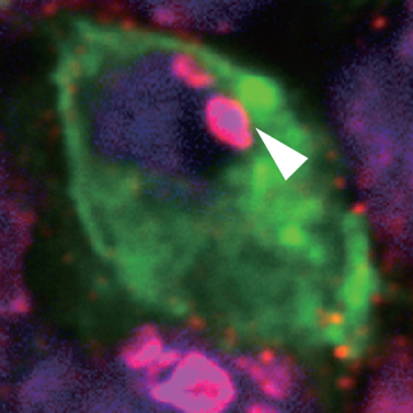 During the migration of primordial germ cells (PGCs) to the genital ridge and during gonadal development, the stepwise erasure of DNA methylation and histone dimethylation marks ensures PGC totipotency and prevents the accumulation of epimutations. On p. 2892, Yoshiyuki Seki and co-workers explore the mechanisms underlying genome-wide epigenetic reprogramming in mouse PGCs by investigating the dynamics of epigenetic modifications in transposable elements. CpG methylation is markedly decreased in short interspersed nuclear elements (SINEs) in migrating PGCs, they report, but not in long interspersed nuclear elements (LINEs). By contrast, CpGs are rapidly demethylated in both SINEs and LINEs in gonadal PGCs. Four major factors that maintain DNA and histone methylation during DNA replication (and whose inhibition is associated with replication-dependent passive demethylation) are repressed at distinct stages of PGC development, they report, and DNA demethylation of transposable elements is disturbed in PGCs in which proliferation is impaired. These and other results suggest that PGCs use both active enzyme-catalysed DNA demethylation and passive demethylation for genome-wide epigenetic reprogramming.
During the migration of primordial germ cells (PGCs) to the genital ridge and during gonadal development, the stepwise erasure of DNA methylation and histone dimethylation marks ensures PGC totipotency and prevents the accumulation of epimutations. On p. 2892, Yoshiyuki Seki and co-workers explore the mechanisms underlying genome-wide epigenetic reprogramming in mouse PGCs by investigating the dynamics of epigenetic modifications in transposable elements. CpG methylation is markedly decreased in short interspersed nuclear elements (SINEs) in migrating PGCs, they report, but not in long interspersed nuclear elements (LINEs). By contrast, CpGs are rapidly demethylated in both SINEs and LINEs in gonadal PGCs. Four major factors that maintain DNA and histone methylation during DNA replication (and whose inhibition is associated with replication-dependent passive demethylation) are repressed at distinct stages of PGC development, they report, and DNA demethylation of transposable elements is disturbed in PGCs in which proliferation is impaired. These and other results suggest that PGCs use both active enzyme-catalysed DNA demethylation and passive demethylation for genome-wide epigenetic reprogramming.
Wnt signalling in early embryos
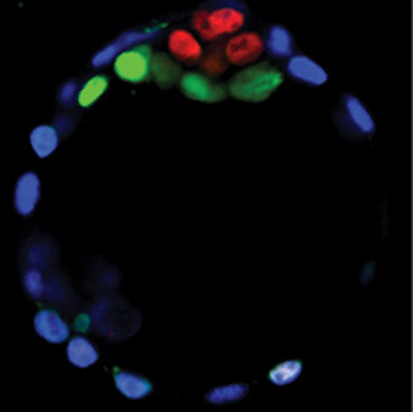 The Wnt signalling pathway is clearly required for gastrulation in mammalian embryos, but little is known about its extra-embryonic and preimplantation functions. Here (p. 2961), Janet Rossant and co-workers investigate the requirements for Wnt signalling in early mouse development using a mouse line that carries a floxed allele for the porcupine homolog (Porcn) gene. Porcn is required for the acylation and secretion of all 19 mammalian Wnt ligands, so Porcn function represents a bottleneck for Wnt signalling. Using zygotic, oocyte-specific and visceral endoderm-specific deletions of Porcn, the researchers show that Porcn-dependent Wnt signalling is not required for preimplantation development or for implantation itself, and they confirm that gastrulation is the first Porcn/Wnt-dependent event in embryonic tissues. They also identify chorio-allantoic fusion as the first major Porcn/Wnt-dependent event in extra-embryonic tissues. Together, these findings show that, although Porcn-dependent Wnt signalling is important for embryonic and placental function, it does not have an essential role in preimplantation development or in blastocyst lineage specification.
The Wnt signalling pathway is clearly required for gastrulation in mammalian embryos, but little is known about its extra-embryonic and preimplantation functions. Here (p. 2961), Janet Rossant and co-workers investigate the requirements for Wnt signalling in early mouse development using a mouse line that carries a floxed allele for the porcupine homolog (Porcn) gene. Porcn is required for the acylation and secretion of all 19 mammalian Wnt ligands, so Porcn function represents a bottleneck for Wnt signalling. Using zygotic, oocyte-specific and visceral endoderm-specific deletions of Porcn, the researchers show that Porcn-dependent Wnt signalling is not required for preimplantation development or for implantation itself, and they confirm that gastrulation is the first Porcn/Wnt-dependent event in embryonic tissues. They also identify chorio-allantoic fusion as the first major Porcn/Wnt-dependent event in extra-embryonic tissues. Together, these findings show that, although Porcn-dependent Wnt signalling is important for embryonic and placental function, it does not have an essential role in preimplantation development or in blastocyst lineage specification.
Gpr125 helps set gastrulation in motion
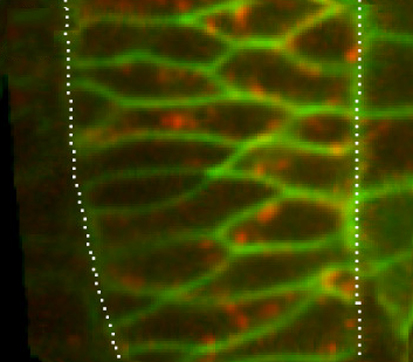 During vertebrate gastrulation, polarised cell behaviours orchestrated by Wnt/planar cell polarity (PCP) signalling drive the convergence and extension (C&E) movements that elongate the embryo. Xin Li, Florence Marlow, Lilianna Solnica-Krezel and colleagues now identify Gpr125, an adhesion G protein-coupled receptor, as a novel modulator of Wnt/PCP signalling during gastrulation in zebrafish embryos (p. 3028). The researchers show that overexpression of Gpr125 impairs C&E movements in zebrafish embryos and that reduced Gpr125 function exacerbates the C&E defects and the facial branchiomotor neuron migration defects seen in embryos with reduced Wnt/PCP signalling. Gpr125 directly interacts with Dishevelled (Dvl), they report, and recruits Dvl to the cell membrane, a prerequisite for Wnt/PCP activation. Finally, they show that Gpr125 and Dvl mutually redistribute into discrete membrane subdomains and recruit a subset of PCP components into membrane subdomains. Thus, the researchers suggest, Gpr125 might act as a component of PCP membrane complexes and as a modulator of Wnt/PCP signalling in vertebrates.
During vertebrate gastrulation, polarised cell behaviours orchestrated by Wnt/planar cell polarity (PCP) signalling drive the convergence and extension (C&E) movements that elongate the embryo. Xin Li, Florence Marlow, Lilianna Solnica-Krezel and colleagues now identify Gpr125, an adhesion G protein-coupled receptor, as a novel modulator of Wnt/PCP signalling during gastrulation in zebrafish embryos (p. 3028). The researchers show that overexpression of Gpr125 impairs C&E movements in zebrafish embryos and that reduced Gpr125 function exacerbates the C&E defects and the facial branchiomotor neuron migration defects seen in embryos with reduced Wnt/PCP signalling. Gpr125 directly interacts with Dishevelled (Dvl), they report, and recruits Dvl to the cell membrane, a prerequisite for Wnt/PCP activation. Finally, they show that Gpr125 and Dvl mutually redistribute into discrete membrane subdomains and recruit a subset of PCP components into membrane subdomains. Thus, the researchers suggest, Gpr125 might act as a component of PCP membrane complexes and as a modulator of Wnt/PCP signalling in vertebrates.
Plus…
Tubulogenesis
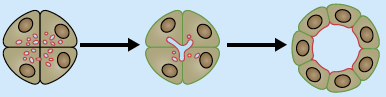 In this poster, Luisa Iruela-Arispe and Greg Beitel summarise our current understanding of the various processes by which tubes form during development, and the cellular and molecular mechanisms underlying tubulogenesis.
In this poster, Luisa Iruela-Arispe and Greg Beitel summarise our current understanding of the various processes by which tubes form during development, and the cellular and molecular mechanisms underlying tubulogenesis.
See the Development at a Glance article on p. 2851
Oct transcription factors in development and stem cells: insights and mechanisms
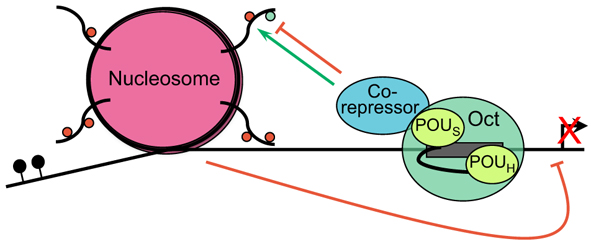 Oct proteins play varied and essential roles during development. Here, Dean Tantin outlines our current understanding of Oct proteins and the regulatory mechanisms that govern their role.
Oct proteins play varied and essential roles during development. Here, Dean Tantin outlines our current understanding of Oct proteins and the regulatory mechanisms that govern their role.
See the Primer article on p. 2857


 (No Ratings Yet)
(No Ratings Yet)