In Development this week (Vol. 140, Issue 24)
Posted by Seema Grewal, on 3 December 2013
Here are the highlights from the current issue of Development:
One-step transdifferentiation
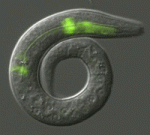 Terminally differentiated cells are generally considered to be in a developmentally locked state in vivo; they are incapable of being directly reprogrammed into an entirely different state. Now, on p. 4844, Joel Rothman and co-workers show that the expression of a single transcription factor can trigger the transdifferentiation of fully differentiated, highly specialised cells in C. elegans larvae and adults. They show that brief ectopic expression of ELT-7, a GATA transcription factor that regulates intestinal differentiation, can specifically convert non-endodermal cells of the pharynx into fully differentiated intestinal cells. This conversion is accompanied by an increase in the expression of intestine-specific genes and a concomitant decrease in the expression of pharynx-specific markers and structural proteins. The reprogrammed cells also exhibit morphological characteristics of intestinal cells. These, together with other findings in the study, demonstrate that terminally differentiated cells can be reprogrammed to an alternative fate without the need for cell division, without the requirement for a dedifferentiated intermediate state and without prior removal of an inhibitory factor.
Terminally differentiated cells are generally considered to be in a developmentally locked state in vivo; they are incapable of being directly reprogrammed into an entirely different state. Now, on p. 4844, Joel Rothman and co-workers show that the expression of a single transcription factor can trigger the transdifferentiation of fully differentiated, highly specialised cells in C. elegans larvae and adults. They show that brief ectopic expression of ELT-7, a GATA transcription factor that regulates intestinal differentiation, can specifically convert non-endodermal cells of the pharynx into fully differentiated intestinal cells. This conversion is accompanied by an increase in the expression of intestine-specific genes and a concomitant decrease in the expression of pharynx-specific markers and structural proteins. The reprogrammed cells also exhibit morphological characteristics of intestinal cells. These, together with other findings in the study, demonstrate that terminally differentiated cells can be reprogrammed to an alternative fate without the need for cell division, without the requirement for a dedifferentiated intermediate state and without prior removal of an inhibitory factor.
Stem cells and regeneration in a new light
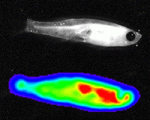 Zebrafish have a remarkable capacity to regenerate and, as such, are being used increasingly to study stem cells and organ regeneration. Here, Chen-Hui Chen, Kenneth Poss and colleagues establish a luciferase-based approach for visualising stem cells and regeneration in adult zebrafish (p. 4988). The researchers generate several transgenic lines that enable ubiquitous or tissue-specific expression of both firefly luciferase and mCherry. They show that, unlike the fluorescence signal, bioluminescence in these lines, which they term zebraflash, readily penetrates through adult tissues and can easily be detected. Using the cardiac zebraflash line, they demonstrate that this approach can be used to monitor the extent of heart injury and subsequent regeneration in animals in a non-invasive and high-throughput manner. Furthermore, they report, this approach can be used to detect quantitatively the progeny of engrafted stem cells in recipient animals at high spatial resolution. This methodology, along with the transgenic lines presented here, offer a valuable resource for the study of stem cells and regeneration.
Zebrafish have a remarkable capacity to regenerate and, as such, are being used increasingly to study stem cells and organ regeneration. Here, Chen-Hui Chen, Kenneth Poss and colleagues establish a luciferase-based approach for visualising stem cells and regeneration in adult zebrafish (p. 4988). The researchers generate several transgenic lines that enable ubiquitous or tissue-specific expression of both firefly luciferase and mCherry. They show that, unlike the fluorescence signal, bioluminescence in these lines, which they term zebraflash, readily penetrates through adult tissues and can easily be detected. Using the cardiac zebraflash line, they demonstrate that this approach can be used to monitor the extent of heart injury and subsequent regeneration in animals in a non-invasive and high-throughput manner. Furthermore, they report, this approach can be used to detect quantitatively the progeny of engrafted stem cells in recipient animals at high spatial resolution. This methodology, along with the transgenic lines presented here, offer a valuable resource for the study of stem cells and regeneration.
Rocking the Wnt pathway
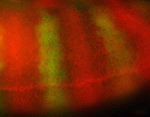 Wnt signalling plays important roles during embryonic patterning and in tissue homeostasis, and mutations that affect the Wnt pathway are associated with cancer. Despite this, the exact way in which the Wnt pathway is regulated is still not fully understood. Now, Amy Bejsovec and colleagues uncover a novel regulator of Wnt signalling (p. 4937). Previous studies have shown that the Drosophila RhoGEF Pebble (Pbl) might influence patterning mediated by Wingless (Wg), the primary fly Wnt. Following this, the authors show that both loss- and gain-of-function Drosophila pbl mutants exhibit defects that are consistent with a role for Pbl in negatively regulating the Wg pathway. Furthermore, both Pbl and ECT2, the human homologue of Pbl, downregulate Wnt reporter activity in cultured Drosophila and human cells, highlighting a role for ECT2 as a potential proto-oncogene. Finally, the researchers show that, unlike most negative regulators of the Wnt pathway, Pbl acts downstream of Armadillo/β-catenin and may act through Rho1 to negatively regulate Wnt/Wg signalling.
Wnt signalling plays important roles during embryonic patterning and in tissue homeostasis, and mutations that affect the Wnt pathway are associated with cancer. Despite this, the exact way in which the Wnt pathway is regulated is still not fully understood. Now, Amy Bejsovec and colleagues uncover a novel regulator of Wnt signalling (p. 4937). Previous studies have shown that the Drosophila RhoGEF Pebble (Pbl) might influence patterning mediated by Wingless (Wg), the primary fly Wnt. Following this, the authors show that both loss- and gain-of-function Drosophila pbl mutants exhibit defects that are consistent with a role for Pbl in negatively regulating the Wg pathway. Furthermore, both Pbl and ECT2, the human homologue of Pbl, downregulate Wnt reporter activity in cultured Drosophila and human cells, highlighting a role for ECT2 as a potential proto-oncogene. Finally, the researchers show that, unlike most negative regulators of the Wnt pathway, Pbl acts downstream of Armadillo/β-catenin and may act through Rho1 to negatively regulate Wnt/Wg signalling.
Opening a passage for hair growth
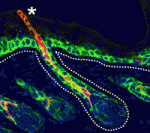 The hair follicle epithelium forms a tube-like structure that is continuous with the epidermis, but how the lumen of this structure is created during morphogenesis and regeneration remains unclear. Now, Sunny Wong and colleagues identify a novel population of cells that initiates hair follicle lumen formation in mice (p. 4870). The researchers first provide a detailed characterisation of the infundibulum, the region encompassing the hair follicle mouth, and identify a population of keratin 79 (K79)-positive epithelial cells within this region. Using lineage tracing, they show that these cells are specified early during hair follicle development and migrate outwards from the hair germ into the epidermis prior to lumen formation. This migratory event is also observed during regeneration of the hair follicle; K79-positive cells are specified in the secondary hair germ and migrate out, eventually forming a continuous layer with pre-existing K79-positive cells. These findings identify both a novel mode of epithelial tube morphogenesis and a unique population of cells that migrate throughout the life cycle of the hair follicle.
The hair follicle epithelium forms a tube-like structure that is continuous with the epidermis, but how the lumen of this structure is created during morphogenesis and regeneration remains unclear. Now, Sunny Wong and colleagues identify a novel population of cells that initiates hair follicle lumen formation in mice (p. 4870). The researchers first provide a detailed characterisation of the infundibulum, the region encompassing the hair follicle mouth, and identify a population of keratin 79 (K79)-positive epithelial cells within this region. Using lineage tracing, they show that these cells are specified early during hair follicle development and migrate outwards from the hair germ into the epidermis prior to lumen formation. This migratory event is also observed during regeneration of the hair follicle; K79-positive cells are specified in the secondary hair germ and migrate out, eventually forming a continuous layer with pre-existing K79-positive cells. These findings identify both a novel mode of epithelial tube morphogenesis and a unique population of cells that migrate throughout the life cycle of the hair follicle.
Insm1 controls pituitary endocrine cell development
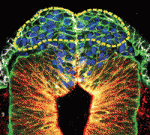
 The pituitary gland is an endocrine organ that plays a role in various physiological processes, including growth, metabolism and reproduction. The development of various pituitary endocrine cells is influenced by a number of transcription factors and signals. In this issue (p. 4947), Carmen Birchmeier and colleagues report that the transcription factor Insm1 controls the differentiation of all endocrine cells in the mouse pituitary. The researchers show that Insm1 is expressed in pituitary progenitors and continues to be expressed in differentiated endocrine cells. Using Insm1 knockout mice, they demonstrate that Insm1 controls a pan-endocrine differentiation programme; genes encoding pituitary hormones or proteins involved in hormone production and secretion are downregulated in Insm1 mutant pituitary glands. By contrast, Notch signalling components and skeletal muscle-specific genes are upregulated, suggesting that Insm1 also represses inappropriate gene expression programmes in the pituitary. Finally, the researchers show that the SNAG domain of Insm1 is required for its function, acting to recruit histone-modifying proteins and transcriptional regulators.
The pituitary gland is an endocrine organ that plays a role in various physiological processes, including growth, metabolism and reproduction. The development of various pituitary endocrine cells is influenced by a number of transcription factors and signals. In this issue (p. 4947), Carmen Birchmeier and colleagues report that the transcription factor Insm1 controls the differentiation of all endocrine cells in the mouse pituitary. The researchers show that Insm1 is expressed in pituitary progenitors and continues to be expressed in differentiated endocrine cells. Using Insm1 knockout mice, they demonstrate that Insm1 controls a pan-endocrine differentiation programme; genes encoding pituitary hormones or proteins involved in hormone production and secretion are downregulated in Insm1 mutant pituitary glands. By contrast, Notch signalling components and skeletal muscle-specific genes are upregulated, suggesting that Insm1 also represses inappropriate gene expression programmes in the pituitary. Finally, the researchers show that the SNAG domain of Insm1 is required for its function, acting to recruit histone-modifying proteins and transcriptional regulators.
Split thoughts on the neural crest
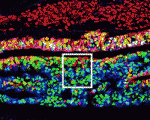
 The neural crest (NC) is a transient structure that gives rise to multiple lineages. Despite intense studies, it is still unclear whether the NC represents a homogeneous population of cells. Here, Jean Paul Thiery and colleagues examine this issue (p. 4890). The authors first characterise the cranial neural fold in chick and mouse embryos and show that, prior to delamination, it contains two phenotypically distinct domains: neural ectoderm and non-neural ectoderm. The researchers then show that the two domains display temporally distinct delamination patterns. Cells specifically within the non-neural ectoderm are the first to delaminate, whereas a second population of delaminating cells then originates from the neural ectoderm in both chick and mouse embryos. Importantly, they report, cells within the two domains have distinct fates: those from the non-neural ectoderm give rise to ectomesenchymal derivatives, whereas those within the neural ectoderm give rise to neuronal derivatives. These, together with other findings, prompt the authors to revisit current definitions of the NC and the origin of ectomesenchyme.
The neural crest (NC) is a transient structure that gives rise to multiple lineages. Despite intense studies, it is still unclear whether the NC represents a homogeneous population of cells. Here, Jean Paul Thiery and colleagues examine this issue (p. 4890). The authors first characterise the cranial neural fold in chick and mouse embryos and show that, prior to delamination, it contains two phenotypically distinct domains: neural ectoderm and non-neural ectoderm. The researchers then show that the two domains display temporally distinct delamination patterns. Cells specifically within the non-neural ectoderm are the first to delaminate, whereas a second population of delaminating cells then originates from the neural ectoderm in both chick and mouse embryos. Importantly, they report, cells within the two domains have distinct fates: those from the non-neural ectoderm give rise to ectomesenchymal derivatives, whereas those within the neural ectoderm give rise to neuronal derivatives. These, together with other findings, prompt the authors to revisit current definitions of the NC and the origin of ectomesenchyme.
PLUS…
Mechanisms of scaling in pattern formation
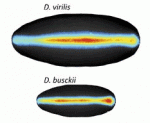 Many organisms and their constituent tissues and organs vary substantially in size but differ little in morphology; they appear to be scaled versions of a common template or pattern. Here, David Umulis and Hans Othmer investigate the underlying principles needed for understanding the mechanisms that can produce scale invariance in spatial pattern formation and discuss examples of systems that scale during development. See the Review article on p. 4830
Many organisms and their constituent tissues and organs vary substantially in size but differ little in morphology; they appear to be scaled versions of a common template or pattern. Here, David Umulis and Hans Othmer investigate the underlying principles needed for understanding the mechanisms that can produce scale invariance in spatial pattern formation and discuss examples of systems that scale during development. See the Review article on p. 4830
Conveying principles of embryonic development by metaphors from daily life
 How can the revolution in our understanding of embryonic development and stem cells be conveyed to the general public? Here, Ben-Zion Shilo presents a photographic approach to highlight scientific concepts of pattern formation using metaphors from daily life, displaying pairs of images of embryonic development and the corresponding human analogy. See the Spotlight article on p. 4827
How can the revolution in our understanding of embryonic development and stem cells be conveyed to the general public? Here, Ben-Zion Shilo presents a photographic approach to highlight scientific concepts of pattern formation using metaphors from daily life, displaying pairs of images of embryonic development and the corresponding human analogy. See the Spotlight article on p. 4827


 (No Ratings Yet)
(No Ratings Yet)