In Development this week (Vol. 141, Issue 23)
Posted by Seema Grewal, on 18 November 2014
Here are the highlights from the new issue of Development:
Akt acts to reprogram germ cells
 Primordial germ cells (PGCs) are unipotent – they go on to form germline stem cells and gametes. However, they are believed to possess latent pluripotency, allowing them to produce the next generation during the normal life-cycle, and to be reprogrammed to pluripotent embryonic germ cells (EGCs) upon experimental manipulation. Various protocols for EGC establishment have been reported, with varying efficiency. Yasuhisa Matsui and colleagues now report a highly efficient method for mouse PGC-to-EGC conversion, using Akt activation in concert with bFGF and LIF treatment (p. 4457). Using a relatively simple protocol starting from purified E10.5 PGCs in culture, the authors are able to achieve 60% reprogramming efficiency, which is significantly higher than any previous method. These reprogrammed EGCs readily contribute to mouse chimeras, including the germline. The authors suggest that Akt may act by inhibiting apoptosis of PGCs and/or by promoting signalling events (including via bFGF and LIF) that mediate PGC-to-EGC reprogramming, thus inducing the latent pluripotency of early PGCs.
Primordial germ cells (PGCs) are unipotent – they go on to form germline stem cells and gametes. However, they are believed to possess latent pluripotency, allowing them to produce the next generation during the normal life-cycle, and to be reprogrammed to pluripotent embryonic germ cells (EGCs) upon experimental manipulation. Various protocols for EGC establishment have been reported, with varying efficiency. Yasuhisa Matsui and colleagues now report a highly efficient method for mouse PGC-to-EGC conversion, using Akt activation in concert with bFGF and LIF treatment (p. 4457). Using a relatively simple protocol starting from purified E10.5 PGCs in culture, the authors are able to achieve 60% reprogramming efficiency, which is significantly higher than any previous method. These reprogrammed EGCs readily contribute to mouse chimeras, including the germline. The authors suggest that Akt may act by inhibiting apoptosis of PGCs and/or by promoting signalling events (including via bFGF and LIF) that mediate PGC-to-EGC reprogramming, thus inducing the latent pluripotency of early PGCs.
Cerebral haemorrhage without leaky vessels
 The vasculature of the central nervous system (CNS) is highly specialised, characterised by the formation of the blood-brain barrier that prevents leakage of vascular contents into the brain. Various molecules and pathways have been implicated in regulating angiogenesis in the CNS, including the αVβ8 integrin and members of the TGFβ pathway. It is thought that αVβ8 integrin expressed in the neuroepithelium regulates TGFβ signalling in the endothelium. On p.4489, Thomas Arnold, Louis Reichardt and colleagues set out to investigate this relationship and the effects of disrupting this signalling cascade on the CNS vasculature. The authors find that disruption of either the integrin or TGFβ pathway components leads to excess angiogenic sprouting, vascular dysplasia and cerebral haemorrhage. Importantly, however, these mutant mice display no signs of compromised blood-brain barrier formation and the vessels, although abnormal, are not leaky. Together, this work defines an important function for αVβ8 integrin-TGFβ signalling in limiting vascular sprouting in the CNS, and demonstrates that cerebral haemorrhage can result from abnormal angiogenesis rather than from blood-brain barrier breakdown.
The vasculature of the central nervous system (CNS) is highly specialised, characterised by the formation of the blood-brain barrier that prevents leakage of vascular contents into the brain. Various molecules and pathways have been implicated in regulating angiogenesis in the CNS, including the αVβ8 integrin and members of the TGFβ pathway. It is thought that αVβ8 integrin expressed in the neuroepithelium regulates TGFβ signalling in the endothelium. On p.4489, Thomas Arnold, Louis Reichardt and colleagues set out to investigate this relationship and the effects of disrupting this signalling cascade on the CNS vasculature. The authors find that disruption of either the integrin or TGFβ pathway components leads to excess angiogenic sprouting, vascular dysplasia and cerebral haemorrhage. Importantly, however, these mutant mice display no signs of compromised blood-brain barrier formation and the vessels, although abnormal, are not leaky. Together, this work defines an important function for αVβ8 integrin-TGFβ signalling in limiting vascular sprouting in the CNS, and demonstrates that cerebral haemorrhage can result from abnormal angiogenesis rather than from blood-brain barrier breakdown.Restricting liver progenitor potential
 The liver possesses a remarkable capacity to regenerate, but what is the source of new cells during regeneration? The two epithelial cell populations – hepatocytes and cholangiocytes – can proliferate upon injury, but there is also evidence for the existence of an adult stem/progenitor cell, the liver progenitor cell (LPC), which resides in or near bile ducts and has both hepatocytic and cholangiocytic potential. Here (p.4448), Naoki Tanimizu and co-workers investigate the abundance and potential of LPCs from neonatal and adult mice. They find that the number of LPCs decreases during postnatal life, and also observe a change in their differentiation potential: adult LPCs are strongly biased towards the cholangiocyte fate and have very limited ability for hepatocytic differentiation, both in vitro and in vivo. Mechanistically, the authors show that the transcription factor grainyhead-like 2 (GRHL2), which is known to promote cholangiocyte differentiation, is more strongly expressed in adult LPCs than neonatal LPCs. GRHL2 in turn inhibits expression of miRNA122, which is important for the hepatocyte lineage. Thus, upregulation of GRHL2 in adult LPCs provides an explanation for their reduced hepatocytic potential, and its inhibition may help to produce functional hepatocytes in the adult liver.
The liver possesses a remarkable capacity to regenerate, but what is the source of new cells during regeneration? The two epithelial cell populations – hepatocytes and cholangiocytes – can proliferate upon injury, but there is also evidence for the existence of an adult stem/progenitor cell, the liver progenitor cell (LPC), which resides in or near bile ducts and has both hepatocytic and cholangiocytic potential. Here (p.4448), Naoki Tanimizu and co-workers investigate the abundance and potential of LPCs from neonatal and adult mice. They find that the number of LPCs decreases during postnatal life, and also observe a change in their differentiation potential: adult LPCs are strongly biased towards the cholangiocyte fate and have very limited ability for hepatocytic differentiation, both in vitro and in vivo. Mechanistically, the authors show that the transcription factor grainyhead-like 2 (GRHL2), which is known to promote cholangiocyte differentiation, is more strongly expressed in adult LPCs than neonatal LPCs. GRHL2 in turn inhibits expression of miRNA122, which is important for the hepatocyte lineage. Thus, upregulation of GRHL2 in adult LPCs provides an explanation for their reduced hepatocytic potential, and its inhibition may help to produce functional hepatocytes in the adult liver.
The origin of the coronary vasculature
 During heart development, the coronary vasculature forms by establishment of an endothelial plexus that expands around the heart. Current evidence suggests that the sinus venosus (SV), endocardium and proepicardium may all contribute to coronary development. However, the relative contributions of these sources and the molecular mechanisms regulating coronary angiogenesis are still unclear. In a detailed lineage-tracing analysis in mouse (p. 4500), Kristy Red-Horse and colleagues find that the SV and endocardium make spatially restricted and complementary contributions to the coronary vasculature, with dorsal and lateral vessels having primarily SV origin, while the endocardium contributes significantly to vessels of the ventral heart and ventricular septum. The proepicardium makes a minor, but non-spatially restricted, contribution. VEGFA has previously been shown to promote angiogenesis of a subpopulation of coronary vasculature, and the authors now find that VEGFC promotes growth of a complementary set of vessels – those derived from the SV. These data provide a comprehensive view of the sources of the coronary endothelium and help to unravel the mechanisms by which these vessels form.
During heart development, the coronary vasculature forms by establishment of an endothelial plexus that expands around the heart. Current evidence suggests that the sinus venosus (SV), endocardium and proepicardium may all contribute to coronary development. However, the relative contributions of these sources and the molecular mechanisms regulating coronary angiogenesis are still unclear. In a detailed lineage-tracing analysis in mouse (p. 4500), Kristy Red-Horse and colleagues find that the SV and endocardium make spatially restricted and complementary contributions to the coronary vasculature, with dorsal and lateral vessels having primarily SV origin, while the endocardium contributes significantly to vessels of the ventral heart and ventricular septum. The proepicardium makes a minor, but non-spatially restricted, contribution. VEGFA has previously been shown to promote angiogenesis of a subpopulation of coronary vasculature, and the authors now find that VEGFC promotes growth of a complementary set of vessels – those derived from the SV. These data provide a comprehensive view of the sources of the coronary endothelium and help to unravel the mechanisms by which these vessels form.PLUS:
An interview with Christopher Wylie and Janet Heasman
 The 2014 Society for Developmental Biology (SDB) Lifetime Achievement Award was jointly awarded to Christopher Wylie and Janet Heasman in recognition of their outstanding and sustained contributions to the field. At the 73rd Annual SDB meeting, where they were presented with the award, we asked Chris and Janet about their careers and their advice for young researchers. See the Spotlight on p. 4415
The 2014 Society for Developmental Biology (SDB) Lifetime Achievement Award was jointly awarded to Christopher Wylie and Janet Heasman in recognition of their outstanding and sustained contributions to the field. At the 73rd Annual SDB meeting, where they were presented with the award, we asked Chris and Janet about their careers and their advice for young researchers. See the Spotlight on p. 4415
How to make a cardiomyocyte
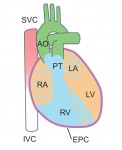 Kenneth Chien and colleagues discuss how insights into the molecular and cellular framework underlying cardiac development can be used to guide the in vitro specification of cardiomyocytes, whether by directed differentiation of pluripotent stem cells or via direct lineage conversion. See the Primer article on p. 4418
Kenneth Chien and colleagues discuss how insights into the molecular and cellular framework underlying cardiac development can be used to guide the in vitro specification of cardiomyocytes, whether by directed differentiation of pluripotent stem cells or via direct lineage conversion. See the Primer article on p. 4418
The cellular and molecular mechanisms of vertebrate lens development
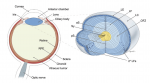 Although lens induction has been studied for over 100 years, recent findings have revealed a myriad of signaling pathways and gene regulatory networks that are required for lens formation in vertebrates. Ales Cvekl and Ruth Ashery-Padan summarize recent progress in the field, emphasizing the interplay between the diverse regulatory mechanisms employed to form lens progenitor and precursor cells. See the Review on p. 4432
Although lens induction has been studied for over 100 years, recent findings have revealed a myriad of signaling pathways and gene regulatory networks that are required for lens formation in vertebrates. Ales Cvekl and Ruth Ashery-Padan summarize recent progress in the field, emphasizing the interplay between the diverse regulatory mechanisms employed to form lens progenitor and precursor cells. See the Review on p. 4432


 (No Ratings Yet)
(No Ratings Yet)