In Development this week (Vol. 141, Issue 4)
Posted by Seema Grewal, on 4 February 2014
Here are the highlights from the current issue of Development:
Integrating integrin signals for neocortical growth
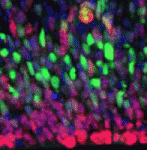 Within the developing neocortex, multiple progenitor cell types contribute to neuronal production, and the properties and relative abundance of these populations help to define the extent of neocortical growth. As well as apically localised radial glial cells (RGCs) that comprise the stem cell compartment, various populations of basal progenitors (BPs) exist – including basal RGCs, with both self-renewal and proliferative capacity, and more restricted progenitors. The stem cell-like properties of RGCs are linked to the fact that these cells are connected to the basal lamina, from which they receive proliferative signals via integrins. Now, Denise Stenzel et al. investigate in rodents the role of integrin αvβ3 in regulating the proliferative capacity of BPs (p. 795). They find that activation of this integrin induces BP proliferation both in hemisphere culture and in vivo, promoting proliferative division at the expense of neurogenic division. Moreover, they show that the αvβ3 ligand thyroid hormone also regulates BP proliferation via this integrin – providing pro-proliferative signals to progenitor cells that are not connected to the basal lamina.
Within the developing neocortex, multiple progenitor cell types contribute to neuronal production, and the properties and relative abundance of these populations help to define the extent of neocortical growth. As well as apically localised radial glial cells (RGCs) that comprise the stem cell compartment, various populations of basal progenitors (BPs) exist – including basal RGCs, with both self-renewal and proliferative capacity, and more restricted progenitors. The stem cell-like properties of RGCs are linked to the fact that these cells are connected to the basal lamina, from which they receive proliferative signals via integrins. Now, Denise Stenzel et al. investigate in rodents the role of integrin αvβ3 in regulating the proliferative capacity of BPs (p. 795). They find that activation of this integrin induces BP proliferation both in hemisphere culture and in vivo, promoting proliferative division at the expense of neurogenic division. Moreover, they show that the αvβ3 ligand thyroid hormone also regulates BP proliferation via this integrin – providing pro-proliferative signals to progenitor cells that are not connected to the basal lamina.
Tracing the origin of epidermal immune cells
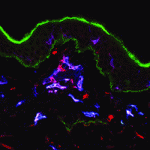 Langerhans cells (LCs) are antigen-presenting cells of the epidermis, and play a key role in detecting pathogens in the skin and coordinating the immune response. However, the precursors of LCs during embryonic development are poorly characterised, particularly in humans. Here (p. 807), Adelheid Elbe-Bürger and colleagues analyse the characteristics of early LC precursors in human first-trimester foetal skin, and explore the potential of different populations of haematopoietic precursors to colonise the developing epidermis. They find an unexpected diversity in LC precursors in terms of cell-surface markers, contrasting with the homogeneous adult LC population. The authors then used in vitro skin equivalent cultures to test the ability of different cord blood-derived precursor populations to colonise the epidermis and differentiate as LCs. Various precursor subtypes show LC potential, and the data suggest that the skin equivalents produce those signals necessary to direct LC differentiation. Together, these data suggest a heterogeneous origin for human embryonic LC precursors during early stages of development.
Langerhans cells (LCs) are antigen-presenting cells of the epidermis, and play a key role in detecting pathogens in the skin and coordinating the immune response. However, the precursors of LCs during embryonic development are poorly characterised, particularly in humans. Here (p. 807), Adelheid Elbe-Bürger and colleagues analyse the characteristics of early LC precursors in human first-trimester foetal skin, and explore the potential of different populations of haematopoietic precursors to colonise the developing epidermis. They find an unexpected diversity in LC precursors in terms of cell-surface markers, contrasting with the homogeneous adult LC population. The authors then used in vitro skin equivalent cultures to test the ability of different cord blood-derived precursor populations to colonise the epidermis and differentiate as LCs. Various precursor subtypes show LC potential, and the data suggest that the skin equivalents produce those signals necessary to direct LC differentiation. Together, these data suggest a heterogeneous origin for human embryonic LC precursors during early stages of development.
How to make a heart: insights from machine learning
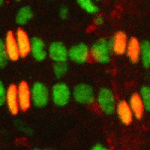 The Drosophila heart provides a relatively simple system for the analysis of gene regulatory networks (GRNs), as it comprises just two cell types – contractile cardial cells (CCs) and non-muscle pericardial cells (PCs). Moreover, many transcription factors (TFs) that regulate Drosophila heart development have been identified and shown to play conserved roles in mammals. On p. 878, Alan Michelson and co-workers take a machine learning approach to identify new cardiac enhancers. The methodology, which integrates chromatin immunoprecipitation data with TF motif mapping, is highly successful at predicting enhancers that drive expression in cardiac cells, including PC- or CC-specific enhancers. Among the highly over-represented TF motifs in putative cardiac enhancers are motifs for the Myb and Su(H) TFs; the authors show that Myb regulates progenitor cell division, while Su(H) controls the PC/CC lineage decision. This work demonstrates the utility of machine learning to identify novel players regulating organ formation, and to piece together the GRNs underlying cell fate specification.
The Drosophila heart provides a relatively simple system for the analysis of gene regulatory networks (GRNs), as it comprises just two cell types – contractile cardial cells (CCs) and non-muscle pericardial cells (PCs). Moreover, many transcription factors (TFs) that regulate Drosophila heart development have been identified and shown to play conserved roles in mammals. On p. 878, Alan Michelson and co-workers take a machine learning approach to identify new cardiac enhancers. The methodology, which integrates chromatin immunoprecipitation data with TF motif mapping, is highly successful at predicting enhancers that drive expression in cardiac cells, including PC- or CC-specific enhancers. Among the highly over-represented TF motifs in putative cardiac enhancers are motifs for the Myb and Su(H) TFs; the authors show that Myb regulates progenitor cell division, while Su(H) controls the PC/CC lineage decision. This work demonstrates the utility of machine learning to identify novel players regulating organ formation, and to piece together the GRNs underlying cell fate specification.
Controlling meristem activity
 The shoot apical meristem (SAM) of higher plants contains the stem cell population that contributes to all above-ground organs. During vegetative growth, leaf primordia emerge from the periphery of the SAM, and the SAM transitions to an inflorescence meristem to initiate the reproductive phase. The WUSCHEL transcription factor specifies stem cell fate, and hence controls the size and activity of the SAM. A gene network involving the CLAVATA signalling pathway, the microRNA miR166g and HD-ZIPIII transcription factors is responsible for regulating WUSCHEL levels. On p. 830, Leor Eshed Williams and colleagues add another layer to this regulatory system, defining a role for the ERECTA receptor kinase in restricting WUSCHEL expression. Plants overexpressing miR166g and carrying a mutation in ERECTA display a massively enlarged SAM, disrupted phyllotaxis patterns and defects in floral meristem formation. ERECTA acts independently of the CLAVATA system, and although the mechanisms by which it functions are not fully clear, this work adds another important player to the complex regulatory network underlying meristem activity.
The shoot apical meristem (SAM) of higher plants contains the stem cell population that contributes to all above-ground organs. During vegetative growth, leaf primordia emerge from the periphery of the SAM, and the SAM transitions to an inflorescence meristem to initiate the reproductive phase. The WUSCHEL transcription factor specifies stem cell fate, and hence controls the size and activity of the SAM. A gene network involving the CLAVATA signalling pathway, the microRNA miR166g and HD-ZIPIII transcription factors is responsible for regulating WUSCHEL levels. On p. 830, Leor Eshed Williams and colleagues add another layer to this regulatory system, defining a role for the ERECTA receptor kinase in restricting WUSCHEL expression. Plants overexpressing miR166g and carrying a mutation in ERECTA display a massively enlarged SAM, disrupted phyllotaxis patterns and defects in floral meristem formation. ERECTA acts independently of the CLAVATA system, and although the mechanisms by which it functions are not fully clear, this work adds another important player to the complex regulatory network underlying meristem activity.
Mcr connects cell connections with innate immunity
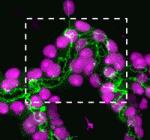 The Drosophila Mcr protein is a member of the thioester protein (TEP) family of proteins, which includes key regulators of innate immunity. Mcr is an unusual member of this family, possessing a putative transmembrane domain and lacking a key residue in the thioester motif. It has, nevertheless, been shown to be involved in phagocytic uptake in cultured Drosophila cells, although its putative immune functions have not been assessed in vivo. Two papers, from Stefan Luschnig and colleagues (p. 899) and Robert Ward and colleagues (p. 889), now identify Mcr as a new component of septate junctions (SJs), the invertebrate equivalent of tight junctions that form the paracellular barrier in epithelia.
The Drosophila Mcr protein is a member of the thioester protein (TEP) family of proteins, which includes key regulators of innate immunity. Mcr is an unusual member of this family, possessing a putative transmembrane domain and lacking a key residue in the thioester motif. It has, nevertheless, been shown to be involved in phagocytic uptake in cultured Drosophila cells, although its putative immune functions have not been assessed in vivo. Two papers, from Stefan Luschnig and colleagues (p. 899) and Robert Ward and colleagues (p. 889), now identify Mcr as a new component of septate junctions (SJs), the invertebrate equivalent of tight junctions that form the paracellular barrier in epithelia.
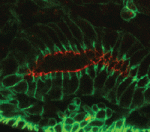 Both studies identify lethal EMS mutations inMcr in independent genetic screens, finding mutant phenotypes typical of SJ components. Consistent with a putative role in SJ formation, the protein colocalises with other SJ proteins at the lateral membrane. They further find that Mcr localisation is dependent on core SJ components and that, conversely, SJ proteins are mislocalised in Mcr mutants. At the morphological and ultrastructural level, SJs are disrupted in the absence of Mcr, and functional assays demonstrate that Mcr is required to form an effective paracellular barrier. As well as identifying a new SJ protein essential for barrier integrity, these two studies suggest an intriguing link between SJs and innate immunity. The epithelial barrier represents the first line of defence against pathogen invasion, andDrosophila haemocytes are known to undergo an epithelialisation-like process when encapsulating pathogens in the haemolymph. The identification of Mcr as a protein involved in both SJ formation and innate immunity now provides a molecular connection, and opens up new avenues for investigating potential functional links, between these two seemingly disparate processes.
Both studies identify lethal EMS mutations inMcr in independent genetic screens, finding mutant phenotypes typical of SJ components. Consistent with a putative role in SJ formation, the protein colocalises with other SJ proteins at the lateral membrane. They further find that Mcr localisation is dependent on core SJ components and that, conversely, SJ proteins are mislocalised in Mcr mutants. At the morphological and ultrastructural level, SJs are disrupted in the absence of Mcr, and functional assays demonstrate that Mcr is required to form an effective paracellular barrier. As well as identifying a new SJ protein essential for barrier integrity, these two studies suggest an intriguing link between SJs and innate immunity. The epithelial barrier represents the first line of defence against pathogen invasion, andDrosophila haemocytes are known to undergo an epithelialisation-like process when encapsulating pathogens in the haemolymph. The identification of Mcr as a protein involved in both SJ formation and innate immunity now provides a molecular connection, and opens up new avenues for investigating potential functional links, between these two seemingly disparate processes.
PLUS…
Cytonemes as specialized signaling filopodia
![]() Thomas Kornberg and Sougate Roy review the evidence that establishes a fundamental and essential role for cytonemes as specialized filopodia that transport signaling proteins between signaling cells. See the Primer article on p. 729
Thomas Kornberg and Sougate Roy review the evidence that establishes a fundamental and essential role for cytonemes as specialized filopodia that transport signaling proteins between signaling cells. See the Primer article on p. 729
Pax genes: regulators of lineage specification and progenitor cell maintenance
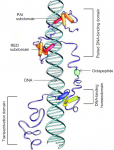 Pax genes encode a family of transcription factors that orchestrate complex processes of lineage determination in the developing embryo. Here, Judith Blake and Melanie Ziman review the molecular functions of Pax genes during development and detail the regulatory mechanisms by which they specify and maintain progenitor cells across various tissue lineages. See the Primer article on p. 737
Pax genes encode a family of transcription factors that orchestrate complex processes of lineage determination in the developing embryo. Here, Judith Blake and Melanie Ziman review the molecular functions of Pax genes during development and detail the regulatory mechanisms by which they specify and maintain progenitor cells across various tissue lineages. See the Primer article on p. 737
How to make an intestine
 James Wells and Jason Spence review how recent advances in developmental and stem cell biology have made it possible to generate complex, three-dimensional, human intestinal tissues in vitro through directed differentiation of human pluripotent stem cells. See the Primer on p. 752
James Wells and Jason Spence review how recent advances in developmental and stem cell biology have made it possible to generate complex, three-dimensional, human intestinal tissues in vitro through directed differentiation of human pluripotent stem cells. See the Primer on p. 752


 (No Ratings Yet)
(No Ratings Yet)